429120
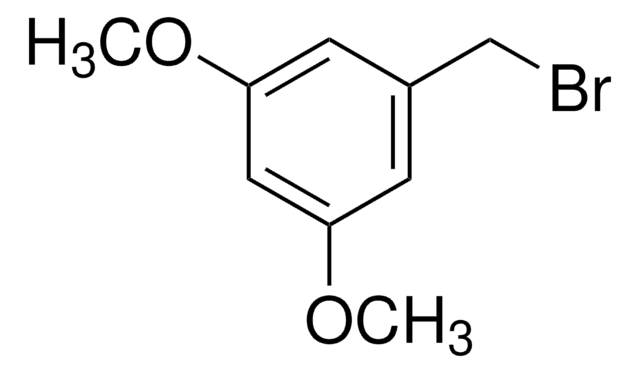
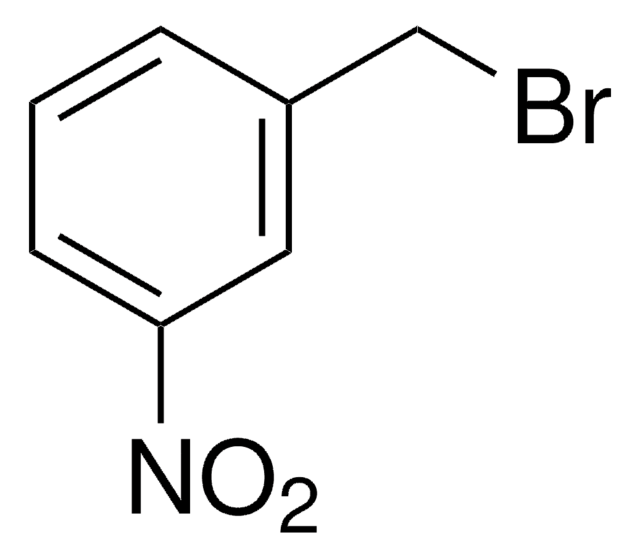
3-Methoxybenzyl bromide
98%
Sinónimos:
1-Bromomethyl-3-methoxybenzene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3OC6H4CH2Br
Número de CAS:
Peso molecular:
201.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.575 (lit.)
bp
152 °C (lit.)
density
1.436 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(CBr)c1
InChI
1S/C8H9BrO/c1-10-8-4-2-3-7(5-8)6-9/h2-5H,6H2,1H3
InChI key
ZKSOJQDNSNJIQW-UHFFFAOYSA-N
General description
3-Methoxybenzyl bromide is a benzyl bromide derivative.
Application
3-Methoxybenzyl bromide (1-bromomethyl-3-methoxybenzene) may be used in the diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 6-(3-methoxyphenyl)-hexane-2,4-dione
- N-(3-methoxybenzyl)-N-(1-methyl-1-phenylethyl)-amine
- 2-(3-methoxybenzyl)-3-[(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]-(3S)-2-thionia-bicyclo [2.2.1]- heptane tetrafluoroborate
- 1-(3-methoxybenzyl)-5-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazole
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Yang Zhang et al.
The Journal of organic chemistry, 71(12), 4516-4520 (2006-06-06)
A mild protocol for the conversion of beta-ketoesters and beta-diketones to carboxylic acids with use of CAN in CH3CN is described. The method is compatible with a number of functional groups, and can generate carboxylic acids under neutral conditions at
Highly diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives: a new general strategy to (R)-?-arylmethyl-?-butyrolactones.
Pohmakotr M, et al.
Tetrahedron Letters, 45(22), 4315-4318 (2004)
Synthesis of (-)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization.
Clayden J, et al.
Tetrahedron, 58(23), 4727-4733 (2002)
Application of sulfur ylide mediated epoxidations in the asymmetric synthesis of ?-hydroxy-d-lactones. Synthesis of a mevinic acid analogue and (+)-prelactone B.
Aggarwal VK, et al.
Tetrahedron Asymmetry, 60(43), 9725-9733 (2004)
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico