424749
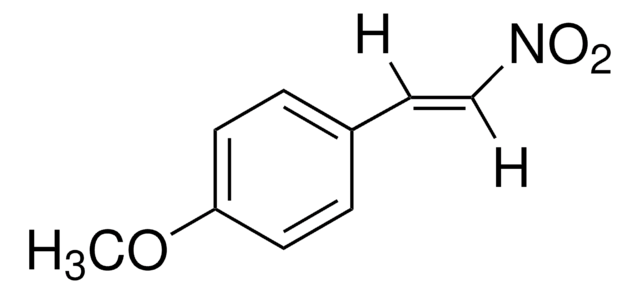
trans-β-Methyl-β-nitrostyrene
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH=C(CH3)NO2
Número de CAS:
Peso molecular:
163.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
solid
mp
63-65 °C (lit.)
functional group
amine
nitro
phenyl
storage temp.
2-8°C
SMILES string
[H]\C(=C(\C)[N+]([O-])=O)c1ccccc1
InChI
1S/C9H9NO2/c1-8(10(11)12)7-9-5-3-2-4-6-9/h2-7H,1H3/b8-7+
InChI key
WGSVFWFSJDAYBM-BQYQJAHWSA-N
Categorías relacionadas
General description
trans-β-Methyl-β-nitrostyrene (1-phenyl-2-nitropropene), a nitrostyrene derivative is an α,β-disubstituted nitroalkene. It has been synthesized by reacting benzaldehyde with nitroethane and butylamine. Spectroscopic analysis of 1-phenyl-2-nitropropene has been done using FT-IR, FT-Raman, NMR and UV.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
A Mori et al.
Chemical & pharmaceutical bulletin, 38(12), 3449-3451 (1990-12-01)
Microbial reduction of 1-phenyl-2-nitro-1-propene (3) was carried out using 57 strains of yeast, 40 strains of aerobic and facultatively anaerobic bacteria and 40 strains of strictly anaerobic bacteria. Nine strains of yeast (Candida tropicalis, etc.,) had the ability to reduce
Gas chromatographic and mass spectral analysis of amphetamine products synthesized from 1-phenyl-2-nitropropene.
DeRuiter J, et al.
Journal of Chromatographic Science, 32(11), 511-519 (1994)
Diego Romano et al.
Microbial cell factories, 13, 60-60 (2014-04-29)
Old Yellow Enzymes (OYEs) are flavin-dependent enoate reductases (EC 1.6.99.1) that catalyze the stereoselective hydrogenation of electron-poor alkenes. Their ability to generate up to two stereocenters by the trans-hydrogenation of the C = C double bond is highly demanded in asymmetric synthesis.
Organocatalytic diastereo- and enantioselective sulfa-Michael addition to α,β-disubstituted nitroalkenes
Pei QL, et al.
Tetrahedron, 69, 5367-5373 (2013)
Tundo P and Andraos J
Green Syntheses, 119-119 (2014)
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 424749-25G | |
| 424749-5G | 4061832103792 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico