384003
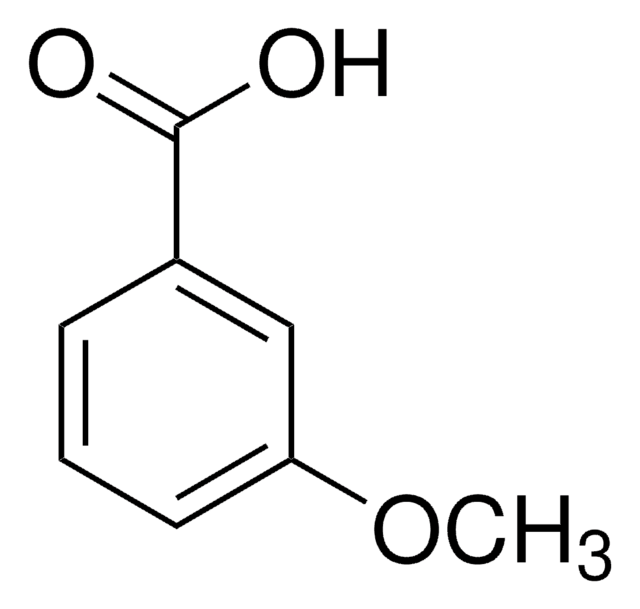
2-Bromo-5-methoxybenzoic acid
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
BrC6H3(OCH3)CO2H
Número de CAS:
Peso molecular:
231.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
solid
mp
157-159 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
COc1ccc(Br)c(c1)C(O)=O
InChI
1S/C8H7BrO3/c1-12-5-2-3-7(9)6(4-5)8(10)11/h2-4H,1H3,(H,10,11)
InChI key
ODHJOROUCITYNF-UHFFFAOYSA-N
Categorías relacionadas
General description
2-Bromo-5-methoxybenzoic acid is a benzoic acid derivative. Synthesis of 2-bromo-5-methoxybenzoic acid and its characterization by HNMR has been reported.
Application
2-Bromo-5-methoxybenzoic acid is suitable for use in the syntheses of urolithin derivatives. It may be used in the synthesis of the following:
- substituted aminobenzacridines
- 8-chloro-2-methoxydibenzo[b,f]thiepin-10(11H)-one and its 3-methoxy derivative
- isoindolinone derivatives
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Lynn S Adams et al.
Cancer prevention research (Philadelphia, Pa.), 3(1), 108-113 (2010-01-07)
Estrogen stimulates the proliferation of breast cancer cells and the growth of estrogen-responsive tumors. The aromatase enzyme, which converts androgen to estrogen, plays a key role in breast carcinogenesis. The pomegranate fruit, a rich source of ellagitannins (ET), has attracted
Design, synthesis and biological evaluation of the novel isoindolinone derivatives.
Liu J, et al.
J. Chem. Res. (M), 6(9), 256-260 (2014)
Potential metabolites of clorotepin: 2-and 3-hydroxy derivatives of 8-chloro-10-(4-methylpiperazino)-10, 11-dihydrodibenzo [b, f] thiepin and their methyl ethers.
Sindelar, K., et al.
Collection of Czechoslovak Chemical Communications, 39(12), 3548-3559 (1974)
Synthesis of substituted aminobenzacridines.
G B BACHMAN et al.
Journal of the American Chemical Society, 68, 1599-1602 (1946-08-01)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)