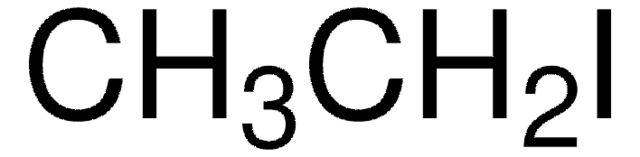362808
Reagent Alcohol
≥89% (GC), reagent grade, contains 5% isopropyl alcohol and 5% methyl alcohol as denaturant
Sinónimos:
Denatured ethanol, Ethyl alcohol, Reagent alcohol, Ethanol, RGA, denatured alcohol
About This Item
Productos recomendados
Nombre del producto
Reagent Alcohol, reagent grade
grade
reagent grade
Quality Level
vapor density
1.59 (vs air)
assay
≥89% (GC)
form
liquid
contains
5% isopropyl alcohol as denaturant
5% methyl alcohol as denaturant
expl. lim.
3.1-27.7 % (lit.)
concentration
100%
dilution
(for analytical testing)
impurities
≤0.1% water
evapn. residue
<0.0005%
bp
78 °C (lit.)
mp
-114 °C (lit.)
application(s)
sample preservation
SMILES string
OCC
InChI
1S/C2H6O/c1-2-3/h3H,2H2,1H3
InChI key
LFQSCWFLJHTTHZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Solvent in organic synthesis (Ex. Esterification reaction)
- Solvent for cytofluorimetric analysis
- Antimicrobial and disinfectant agent
- Cleaning
- Purification
- General Chemical Syntheses
Features and Benefits
Components
Replacement part
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 2
target_organs
Eyes,Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
48.2 °F - closed cup
flash_point_c
9 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Protocolos
While quantitative analysis was performed for Vitamins D2 and D3, the samples were scanned for the presence of the 25-hydroxy metabolites.
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 362808-1L | 4061831824865 |
| 362808-200L-P1 | 4061838161574 |
| 362808-4L | 4061831824872 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico