357839
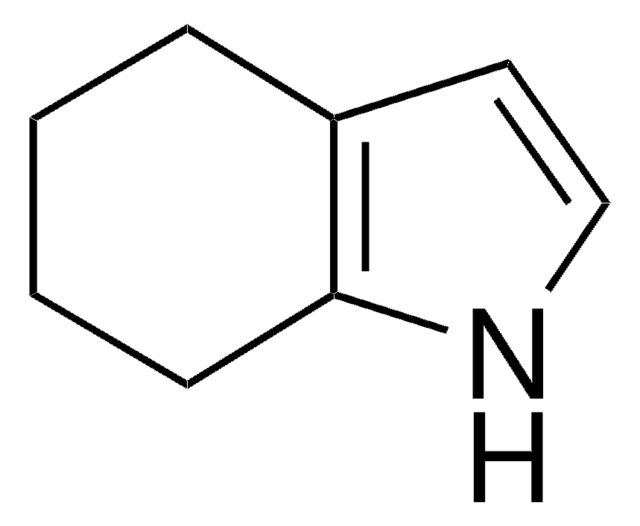
1,5,6,7-Tetrahydro-4H-indol-4-one
98%
Sinónimos:
4,5,6,7-Tetrahydro-4-oxoindole, NSC 131681
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H9NO
Número de CAS:
Peso molecular:
135.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
mp
188-190 °C (lit.)
functional group
ketone
SMILES string
O=C1CCCc2[nH]ccc12
InChI
1S/C8H9NO/c10-8-3-1-2-7-6(8)4-5-9-7/h4-5,9H,1-3H2
InChI key
KASJZXHXXNEULX-UHFFFAOYSA-N
General description
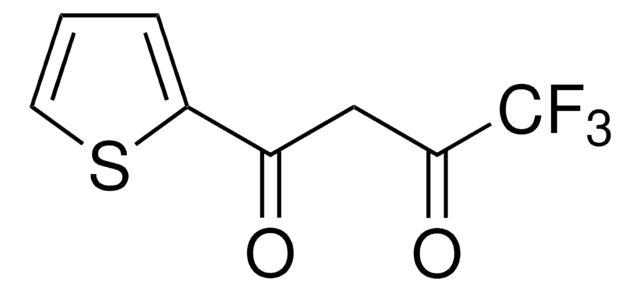
Iodination of 1,5,6,7-tetrahydro-4H-indol-4-one using 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) yields α-iodo derivative as the main product.
Application
1,5,6,7-Tetrahydro-4H-indol-4-one may be used in the preparation of:
- 4-oxo-4,5,6,7-tetrahydro-1H-indole-2-carbonitrile
- methyl 2-(4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)acrylate
- 3-(4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)propanenitrile
- potent and orally active 5-HT1A agonists, (R)-(+)- and (S)-(-)-1-formyl-6,7,8,9-tetrahydro-N,N-dipropyl-3H-benz[e]indol-8-amines
- Reactant in synthesis of psammopemmin A as antitumor agent
- Reactant in synthesis of a 1,3,4,5-tetrahydrobenzindole β-ketoesters and tricyclic tetrahydrobenzindoles via C-H insertion reactions
- Reactant in preparation of tricyclic indole and dihydroindole derivatives as inhibitors of guanylate cyclase
- Reactant in preparation of condensed pyrroloindoles via Pd-catalyzed intramolecular C-H bond functionalization of (halobenzyl)pyrroles
- Reactant in enantioselective preparation of arylalkenyl indoles via asymmetric C-H insertion of rhodium carbenoids followed by Cope rearrangement-elimination
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
DNA binders: 1. evaluation of DNA-interactive ability, design, and synthesis of novel intercalating agents
Sechi M, et al.
Letters in Drug Design & Discovery, 6(1), 56-62 (2009)
Direct α-iodination of aryl alkyl ketones by elemental iodine activated by 1-chloromethyl-4-fluoro-1, 4-diazoniabicyclo [2.2. 2] octane bis (tetrafluoroborate).
Jereb M, et al.
Synthesis, 06, 0853-0858 (2003)
Synthesis of pyrrolo [1, 2-a] indole-1, 8 (5H)-diones as new synthons for developing novel tricyclic compounds of pharmaceutical interest.
Sechi M, et al.
ARKIVOC (Gainesville, FL, United States), 5, 97-106 (2004)
Synthesis of (R)-and (S)-1-formyl-6, 7, 8, 9-tetrahydro-N, N-(dipropyl)-3H-benz [e] indol-8-amines: potent and orally active 5-HT1A receptor agonists.
Lin C-H, et al.
Journal of Heterocyclic Chemistry, 31(1), 129-139 (1994)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico