357804
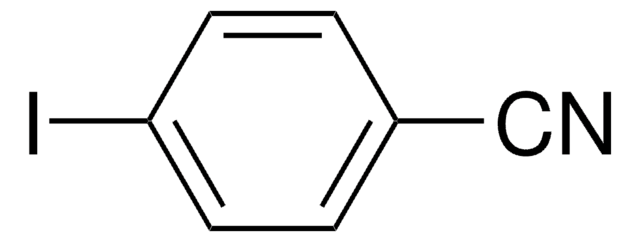
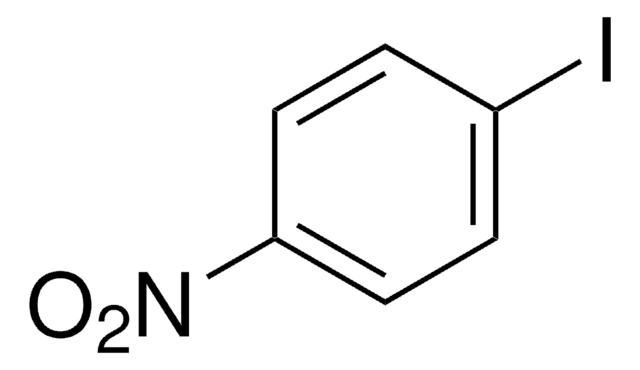
4′-Iodoacetophenone
≥97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
IC6H4COCH3
Número de CAS:
Peso molecular:
246.05
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
≥97%
mp
82-84 °C (lit.)
functional group
iodo
ketone
SMILES string
CC(C1=CC=C(I)C=C1)=O
InChI
1S/C8H7IO/c1-6(10)7-2-4-8(9)5-3-7/h2-5H,1H3
InChI key
JZJWCDQGIPQBAO-UHFFFAOYSA-N
General description
Pd(0)-catalyzed cross coupling reaction of 4′-iodoacetophenone with siloxane has been reported. Heck-Mizoroki reactions of 4′-iodoacetophenone with styrene catalyzed by Pd nanoparticles in the flow reactor has been reported.
Application
4′-Iodoacetophenone may be used as substrate for the palladium-catalyzed coupling reactions. It may be used in the synthesis of quinoline-based potential anticancer agents.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
G J Atwell et al.
Journal of medicinal chemistry, 32(2), 396-401 (1989-02-01)
A series of phenyl-substituted derivatives of the "minimal" DNA-intercalating agent N-[2-(dimethylamino)-ethyl]-2-phenylquinoline-8-carboxamide (1) have been synthesized and evaluated for in vivo antitumor activity, in a continuing search for active compounds of this class with the lowest possible DNA association constants. Substitution
S E Denmark et al.
Organic letters, 3(11), 1749-1752 (2001-06-19)
A sequential ring-closing metathesis/silicon-assisted cross-coupling sequence has been developed. Alkenyldimethylsilyl ethers of omega-unsaturated alcohols undergo facile ring closure with Schrock's catalyst to afford five-, six-, and seven-membered cycloalkenylsiloxanes bearing substituents on both alkenyl carbons. These siloxanes were highly effective coupling
Klaas Mennecke et al.
Beilstein journal of organic chemistry, 5, 21-21 (2009-07-11)
The preparation of monolithic polyionic supports which serve as efficient heterogeneous supports for palladium(0) nanoparticles is described. These functionalized polymers were incorporated inside a flow reactor and employed in Suzuki-Miyaura and Heck cross couplings under continuous flow conditions.
Chemistry Letters (Jpn), 2049-2049 (1989)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico