349593
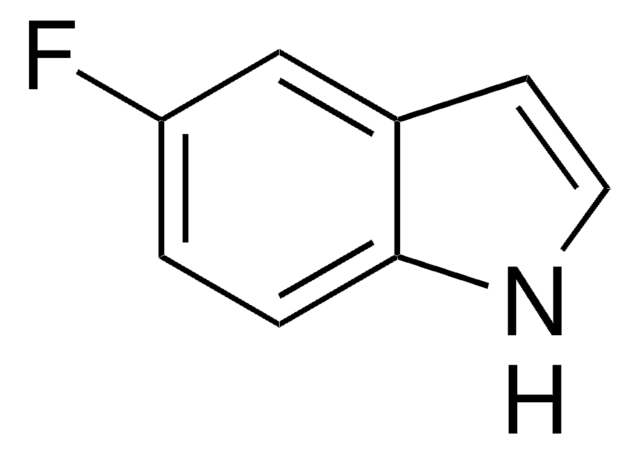
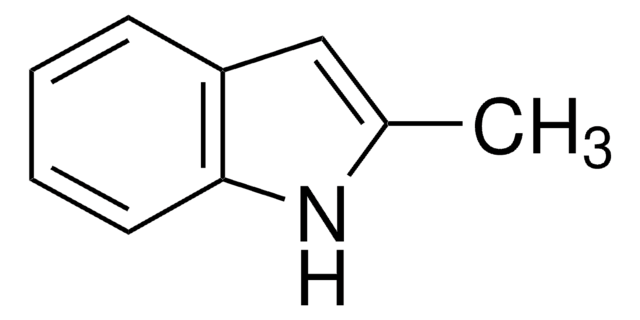
6-Fluoroindole
98%
Sinónimos:
NSC 520436
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H6FN
Número de CAS:
Peso molecular:
135.14
Beilstein/REAXYS Number:
112192
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
mp
72-76 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccc2cc[nH]c2c1
InChI
1S/C8H6FN/c9-7-2-1-6-3-4-10-8(6)5-7/h1-5,10H
InChI key
YYFFEPUCAKVRJX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
6-Fluoroindole is a halogen substituted indole. Experimental ionization potential of 6-fluoroindole has been evaluated. Preparation of 6-fluoroindole via nitration of indoline has been reported.
Application
6-Fluoroindole may be used as reactant in the preparation of:
- tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- antibacterial agents
- antifungal agents
- Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the management of hyperglycemia in diabetes
- potent selective serotonin reuptake inhibitors
- inhibitors of HIV-1 attachment
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
G S Sheppard et al.
Journal of medicinal chemistry, 37(13), 2011-2032 (1994-06-24)
(2RS,4R)-3-(2-(3-Pyridinyl)thiazolidin-4-oyl)indoles represent a new class of potent, orally active antagonists of platelet activating factor (PAF). The compounds were prepared by acylation of the magnesium or zinc salts of substituted indoles with (2RS,4R)-2-(3-pyridinyl)-3-(tert-butoxycarbonyl)thiazolidin-4-oyl chloride. The 3-acylindole moiety functions as a hydrolytically
David F Cummings et al.
Bioorganic & medicinal chemistry, 18(13), 4783-4792 (2010-06-24)
Efforts to develop ligands that distinguish between clinically relevant 5-HT2A and 5-HT2C serotonin receptor subtypes have been challenging, because their sequences have high homology. Previous studies reported that a novel aplysinopsin belonging to a chemical class of natural products isolated
Na, Y. M.
Bull. Korean Chem. Soc., 31, 3467-3467 (2010)
S Jimmy Budiardjo et al.
ACS synthetic biology, 5(12), 1475-1484 (2016-07-09)
Chemical biology has long sought to build protein switches for use in molecular diagnostics, imaging, and synthetic biology. The overarching challenge for any type of engineered protein switch is the ability to respond in a selective and predictable manner that
Chun-Hsu Yao et al.
Journal of medicinal chemistry, 54(1), 166-178 (2010-12-07)
A novel series of N-linked β-D-xylosides were synthesized and evaluated for inhibitory activity against sodium-dependent glucose cotransporter 2 (SGLT2) in a cell-based assay. Of these, the 4-chloro-3-(4-cyclopropylbenzyl)-1-(β-D-xylopyranosyl)-1H-indole 19m was found to be the most potent inhibitor, with an EC(50) value
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico