260517
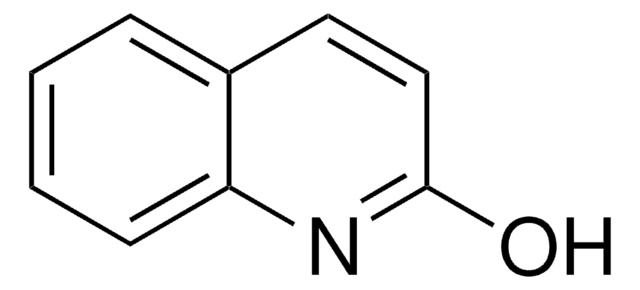
2-Quinoxalinol
99% (HPLC)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
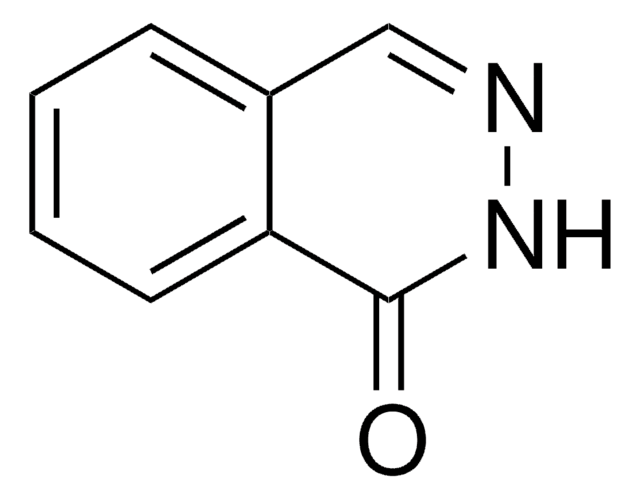
C8H6N2O
Número de CAS:
Peso molecular:
146.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99% (HPLC)
mp
271-272 °C (lit.)
SMILES string
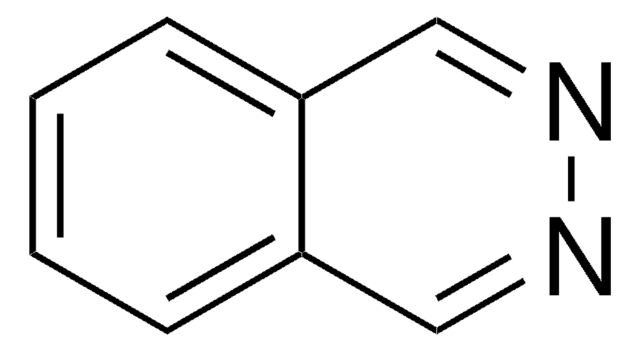
Oc1cnc2ccccc2n1
InChI
1S/C8H6N2O/c11-8-5-9-6-3-1-2-4-7(6)10-8/h1-5H,(H,10,11)
InChI key
FFRYUAVNPBUEIC-UHFFFAOYSA-N
General description
Epitaxial crystallization of syndiotactic polypropylene on 2-quinoxalinol yields isochiral form II of syndiotactic polypropylene. 2-Quinoxalinol participates in direct dehydrative cross-coupling of 2-quinoxalinone with p-tolylacetylene via Pd/Cu-catalyzed phosphonium coupling.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Pramila Menon et al.
Chemosphere, 53(8), 1023-1031 (2003-09-25)
The dissipation of 14C carbaryl in undisturbed soil cores, and of quinalphos (25EC and 20AF) after seed and soil treatments, was investigated under field use conditions, in a semi-arid groundnut field. Residues were analyzed by TLC and HPLC and additionally
R Q He et al.
Biochemistry and molecular biology international, 37(3), 447-457 (1995-10-01)
A procedure for transaminating proteins and removing the transaminated N-terminal residue has been used for studying structure-function relationship of protein (Dixon and Fields 1972, Meth. Enzymol. 25, 409-419). We show that it is convenient for measuring the relative molecular masses
Isochiral form II of syndiotactic polypropylene produced by epitaxial crystallization.
Zhang J, et al.
Macromolecules, 34(18), 6261-6267 (2001)
Fu-An Kang et al.
Chemical communications (Cambridge, England), 46(8), 1347-1349 (2010-05-08)
The first chemoselective direct dehydrative cross-coupling of tautomerizable heterocycles with alkynes has been achieved via C-H/C-OH bond activations with direct C(sp(2))-C(sp) bond formation, which is in line with ideal synthesis using readily available materials.
Andreas Behrends et al.
Redox report : communications in free radical research, 9(5), 279-288 (2004-12-21)
Toxicity of the pesticide quinalphos may comprise secondary, delayed effects by its main metabolite 2-hydroxyquinoxaline (HQO). We demonstrate that HQO can destroy photocatalytically vitamins C and E, catecholamines, serotonin, melatonin, the melatonin metabolite AMK (N(1)-acetyl-5-methoxykynuramine), and unsubstituted and substituted anthranilic
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico