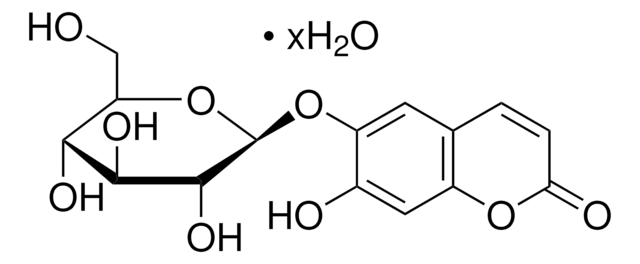
246573
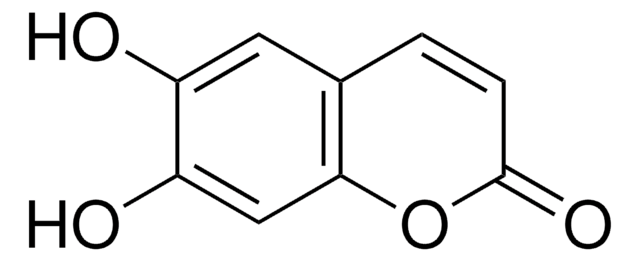
6,7-Dihydroxycoumarin
98%
Sinónimos:
Cichorigenin, Esculetin
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H6O4
Número de CAS:
Peso molecular:
178.14
Beilstein/REAXYS Number:
152788
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Productos recomendados
Quality Level
assay
98%
form
powder
mp
271-273 °C (lit.)
SMILES string
Oc1cc2OC(=O)C=Cc2cc1O
InChI
1S/C9H6O4/c10-6-3-5-1-2-9(12)13-8(5)4-7(6)11/h1-4,10-11H
InChI key
ILEDWLMCKZNDJK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chen Wang et al.
Journal of neurochemistry, 121(6), 1007-1013 (2012-03-31)
Previous studies have demonstrated that a natural coumarin compound esculetin (Esc) possesses antioxidant, anti-tumor, and anti-inflammation activities and rescues cultured primary neurons from NMDA toxicity. In this study, we investigated the neuroprotective effects of Esc on cerebral ischemia/reperfusion (I/R) injury
Aline Witaicenis et al.
Chemico-biological interactions, 186(2), 211-218 (2010-04-13)
Coumarins comprise a broad class of phenolic compounds that influences the formation and scavenging of reactive oxygen species and the processes involving free radical-mediated injury. In light of the antioxidant and anti-inflammatory properties of esculetin and 4-methylesculetin, the aim of
Sudhakar R Subramaniam et al.
Toxicology and applied pharmacology, 250(2), 130-136 (2010-10-12)
Esculetin (6,7-dihydroxy coumarin), is a potent antioxidant that is present in several plant species. The aim of this study was to investigate the mechanism of protection of esculetin in human hepatoma HepG2 cells against reactive oxygen species (ROS) induced by
Li-Wen Tian et al.
Journal of natural products, 72(6), 1057-1060 (2009-05-09)
Five new 7-O-methylkaempferol and -quercetin glycosides, namely, nervilifordins A-E (1-5), were isolated from the whole plant of Nervilia fordii, together with seven known flavonoids (6, 7, and 9-13) and one known coumarin (8). Their structures were elucidated on the basis
Eun-Sun Yun et al.
Toxicology in vitro : an international journal published in association with BIBRA, 25(7), 1335-1342 (2011-05-24)
The phenolic compound esculetin is known to inhibit the proliferation of vascular smooth muscle cells (VSMC). However, the signaling pathway by which esculetin mediates its molecular effects in VSMC remains to be identified. The present results suggest an unexpected role
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico