211605
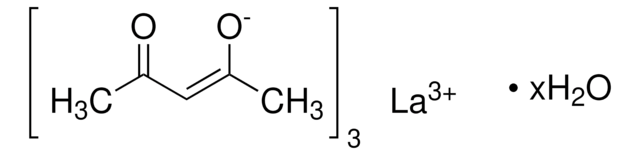
Lanthanum(III) chloride hydrate
99.9% trace metals basis
Sinónimos:
Lanthanum trichloride hydrate, Trichlorolanthanum hydrate
About This Item
Productos recomendados
Quality Level
assay
99.9% trace metals basis
form
powder, crystals or chunks
composition
Degree of hydration, ~6
reaction suitability
reagent type: catalyst
core: lanthanum
impurities
≤1500.0 ppm Trace Rare Earth Analysis
mp
92-93 °C (lit.)
SMILES string
O.Cl[La](Cl)Cl
InChI
1S/3ClH.La.H2O/h3*1H;;1H2/q;;;+3;/p-3
InChI key
NUXZAAJDCYMILL-UHFFFAOYSA-K
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- As a precursor to synthesize La-based nanostructures.
- As a dopant to fabricate ZnO nanoparticles with enhanced optical particles.
- To synthesize Fe–La composite (hydr)oxides for arsenic removal from water.
- Tofabricate lanthanum hexaboride (LaB6) powders forultra-high-temperature ceramics.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico