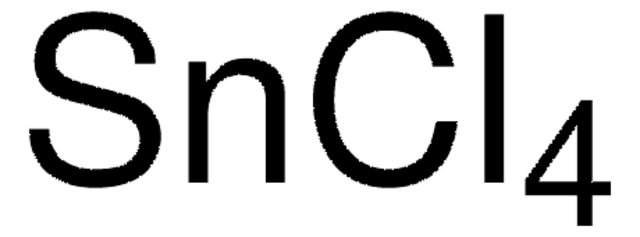208930
Tin(IV) chloride
98%
Sinónimos:
Stannic chloride fuming
About This Item
Productos recomendados
vapor density
9 (vs air)
Quality Level
vapor pressure
10 mmHg ( 10 °C)
18.6 mmHg ( 20 °C)
20 mmHg ( 22 °C)
assay
98%
form
liquid
reaction suitability
core: tin
reagent type: Lewis acid
reagent type: catalyst
bp
114 °C (lit.)
mp
−33 °C (lit.)
density
2.226 g/mL at 25 °C (lit.)
SMILES string
Cl[Sn](Cl)(Cl)Cl
InChI
1S/4ClH.Sn/h4*1H;/q;;;;+4/p-4
InChI key
HPGGPRDJHPYFRM-UHFFFAOYSA-J
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Cyclization of trans β-monocyclohomofarnesic acid to form norambreinolide.
- Formation of C-glycosides of aromatic compounds such as thiophene, naphthalene and phloroglucinol trimethyl ether.
- Formal [4+2] cycloaddition of 3-ethoxycyclobutanones and allyltrialkylsilanes to form 3-ethoxy-5-[(trialkylsilyl)methyl]cyclohexan-1-ones.
- Diastereoselective reaction of δ-alkoxyallylstannanes with aldehydes to form 1,5-diol derivatives.
Other applications of SnCl4:
- The SnCl4-2,6-dialkoxyphenols complexes can catalyze cyclization of poylenes, such as 4-(homogeranyl)toluene to form trans-fused tricyclic compound.
- SnCl4 can act as a promoter during the reaction of ortho-aminobenzonitriles with β-ketoesters and β-enaminonitriles to form 4-aminoquinolines and 4-aminopyridines, respectively.
- SnCl4-silver perchlorate forms an effective catalytic system for the stereoselective of glycosylation 1-O-acetyl glucose.
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico