206490
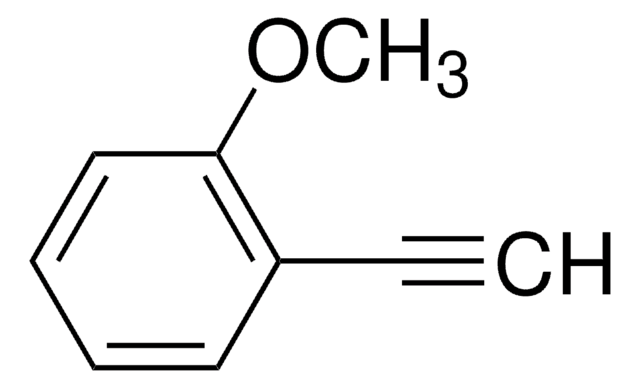
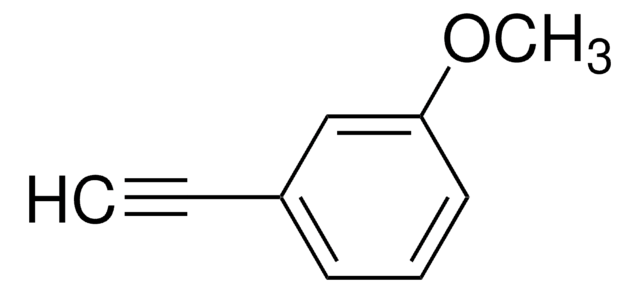
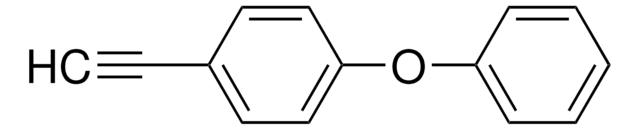
4-Ethynylanisole
97%
Sinónimos:
1-Ethynyl-4-methoxybenzene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
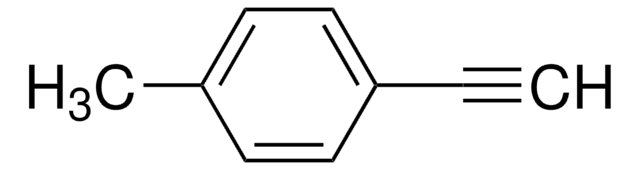
CH3OC6H4C≡CH
Número de CAS:
Peso molecular:
132.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
solid
refractive index
n20/D 1.563 (lit.)
bp
87-91 °C/11 mmHg (lit.)
mp
28-29 °C (lit.)
density
1.019 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(cc1)C#C
InChI
1S/C9H8O/c1-3-8-4-6-9(10-2)7-5-8/h1,4-7H,2H3
InChI key
KBIAVTUACPKPFJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
4-Ethynylanisole forms the corresponding propargyl aldehyde in good yield directly from DMF-dimethyl acetal without the need for making an acetylide salt. 1,3-dipolar cycloaddition of acceptor-cyclopropylmethylsilanes affords functionalized cyclopentenes in good yields.
4-Ethynylanisole can be used as a building block in organic synthesis and also used in sonogashira coupling reactions.
4-Ethynylanisole can be used as a building block in organic synthesis and also used in sonogashira coupling reactions.
Application
1,3-dipolar cycloaddition of acceptor-cyclopropylmethylsilanes affords functionalized cyclopentenes in good yields.
4-Ethynylanisole was used:
- in the synthesis of photoluminescent 1,2-dihydrophosphinines via a [4 + 2] cycloaddition
- along with an arylboronic acid and sodium azide in a copper-catalyzed, three-component synthesis of trisubstituted 1,2,4-triazoles
- in a study of a gold (III)-catalyzed hydroamination of alkynes leading to N-vinylindoles.
Forms the corresponding propargyl aldehyde in good yield directly from DMF-dimethyl acetal without the need for making an acetylide salt.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
179.6 °F - closed cup
flash_point_c
82 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synlett, 278-278 (2007)
Formal [3+2] addition of acceptor-substituted cyclopropylmethylsilanes with aryl acetylenes.
Veejendra K Yadav et al.
Angewandte Chemie (International ed. in English), 43(20), 2669-2671 (2008-07-17)
Laura C Pavelka et al.
Dalton transactions (Cambridge, England : 2003), 41(11), 3294-3301 (2012-01-31)
The addition of 4-trifluoromethyl-1-ethynylbenzene, phenylacetylene, or 4-ethynylanisole to P-mesityldiphenylmethylenephosphine, 1, produced photoluminescent 1,2-dihydrophosphinines 4a-c, respectively, in quantitative yield via a [4 + 2] cycloaddition. Limited reactivity was observed between 1 and non-aromatic alkynes. P-[Bis(trimethylsilyl)amino][(trimethylsilyl)methylene]phosphine, 2, and P-mesityl[(t-butyl)(trimethylsiloxy)methylene]phosphine, 3, showed extremely
Tetrahedron Letters, 45, 5043-5043 (2004)
Yuhua Zhang et al.
Organic letters, 9(4), 627-630 (2007-02-09)
A highly efficient double-hydroamination reaction of o-alkynylanilines with terminal alkynes leading to N-alkenylindoles was developed by using gold(III) as a catalyst under neat conditions. [reaction: see text].
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico