195030
Triethylborane solution
1.0 M in hexanes
Sinónimos:
Triethylboron
About This Item
Productos recomendados
form
liquid
reaction suitability
reagent type: reductant
concentration
1.0 M in hexanes
density
0.675 g/mL at 25 °C
SMILES string
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
LALRXNPLTWZJIJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
- As a radical initiator and terminator of free-radical reactions in aqueous media.(1)
- To synthesize polymers such as poly(2-substituted-1-propenylene)s by reacting with 2-substituted allylic arsonium ylides.(2)
signalword
Danger
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1A - STOT RE 1 Inhalation - STOT SE 3
target_organs
Central nervous system, Nervous system
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
-32.8 °F
flash_point_c
-36 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico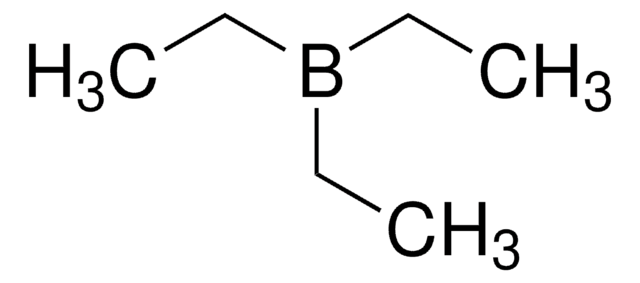




![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)





