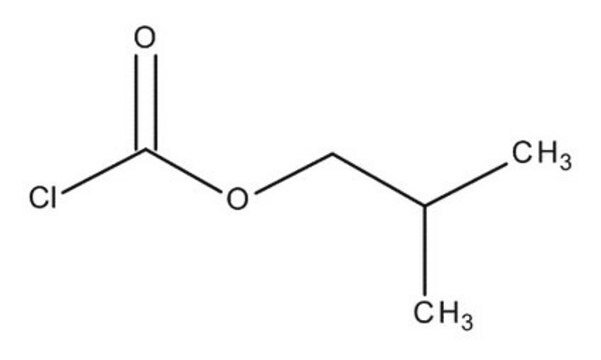
177989
Isobutyl chloroformate
98%
Sinónimos:
Chloroformic acid isobutyl ester, IBCF
About This Item
Productos recomendados
vapor pressure
0.33 mmHg ( 20 °C)
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
128.8 °C (lit.)
solubility
benzene: miscible
chloroform: miscible
diethyl ether: miscible
density
1.053 g/mL at 25 °C (lit.)
SMILES string
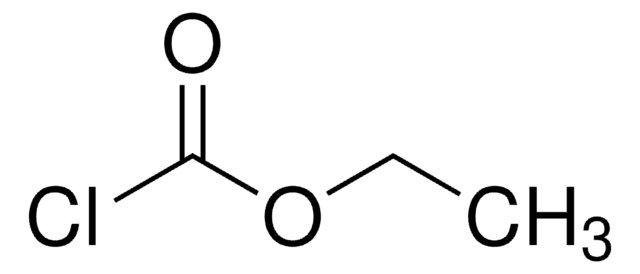
CC(C)COC(Cl)=O
InChI
1S/C5H9ClO2/c1-4(2)3-8-5(6)7/h4H,3H2,1-2H3
InChI key
YOETUEMZNOLGDB-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Preparation of a volatile derivative of taurine and application to gas chromatographic determination of urinary taurine.: Demonstrates the utility of isobutyl chloroformate in preparing volatile derivatives of taurine for gas chromatographic analysis, offering a methodological advancement in clinical biochemistry (Masuoka et al., 1989).
- Quinazoline antifolates inhibiting thymidylate synthase: synthesis of four oligo(L-gamma-glutamyl) conjugates of N10-propargyl-5,8-dideazafolic acid and their enzyme inhibition.: This article investigates the synthesis and biological activity of quinazoline antifolates, using techniques including isobutyl chloroformate, relevant in medicinal chemistry and drug development (Pawelczak et al., 1989).
- Coupling of peptides to protein carriers by mixed anhydride procedure.: Discusses a novel technique using isobutyl chloroformate for peptide coupling to proteins, useful in bioconjugate chemistry and vaccine development (Samokhin & Filimonov, 1985).
- Folate analogues altered in the C9-N10 bridge region. 18. Synthesis and antitumor evaluation of 11-oxahomoaminopterin and related compounds.: Investigates the role of isobutyl chloroformate in synthesizing folate analogues for cancer research, showing its importance in therapeutic chemistry (Nair et al., 1981).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
96.8 °F - closed cup
flash_point_c
36 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico