155721
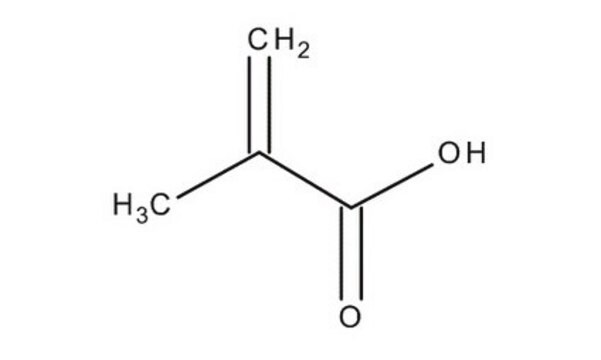
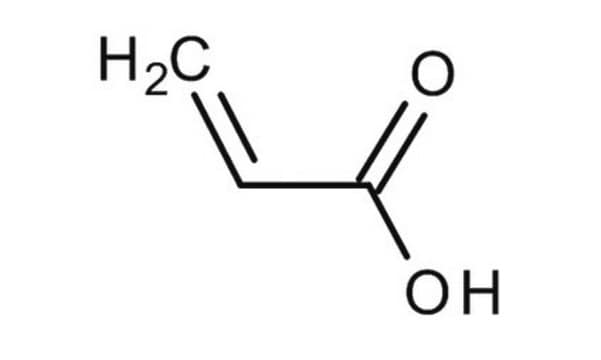
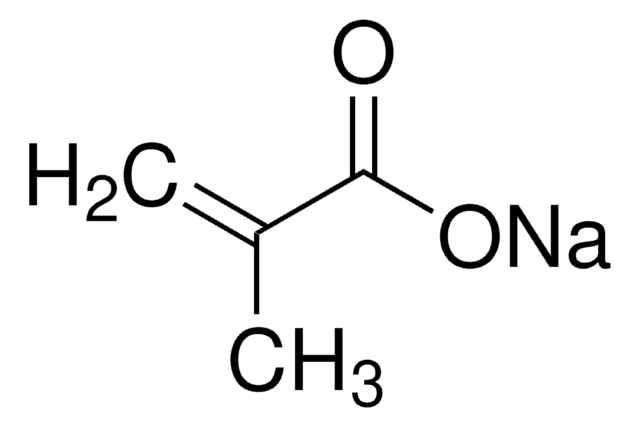
Methacrylic acid
contains 250 ppm MEHQ as inhibitor, 99%
Sinónimos:
2-Methacrylic acid, 2-Methylpropenoic acid
About This Item
Productos recomendados
vapor density
>3 (vs air)
Quality Level
vapor pressure
1 mmHg ( 20 °C)
assay
99%
form
liquid
autoignition temp.
752 °F
contains
250 ppm MEHQ as inhibitor
refractive index
n20/D 1.431 (lit.)
pH
2.0-2.2 (20 °C, 100 g/L)
bp
163 °C (lit.)
mp
12-16 °C (lit.)
density
1.015 g/mL at 25 °C (lit.)
SMILES string
C=C(C)C(O)=O
InChI
1S/C4H6O2/c1-3(2)4(5)6/h1H2,2H3,(H,5,6)
InChI key
CERQOIWHTDAKMF-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
flash_point_f
152.6 °F - closed cup
flash_point_c
67 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
(RAFT) Polymerization
Composites
Artículos
RAFT (Reversible Addition Fragmentation chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
The manufacture of monomers for use in ophthalmic applications is driven by the need for higher purity, improved reliability of manufacturing supply, but ultimately by the need for the increased comfort, convenience, and safety of contact lens wearers. Daily wear contact lenses have the potential to fill this need for many customers; however, their widespread use is constrained by higher costs compared to weekly- or monthly-based lenses. New approaches that improve cost structure and result in higher quality raw materials are needed to help make contact lenses more affordable and accelerate growth of the contact lens market.
By altering the physicochemical properties, smart or intelligent drug delivery systems can be designed to deliver therapeutic molecules on-demand. Learn more about the application of stimuli-responsive materials in drug delivery.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico