151653
Methyl hydrazinocarboxylate
97%
Sinónimos:
Methoxycarbonylhydrazine, Methyl carbazate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
H2NNHCO2CH3
Número de CAS:
Peso molecular:
90.08
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
bp
108 °C/12 mmHg (lit.)
mp
70-73 °C (lit.)
functional group
amine
hydrazine
SMILES string
COC(=O)NN
InChI
1S/C2H6N2O2/c1-6-2(5)4-3/h3H2,1H3,(H,4,5)
InChI key
WFJRIDQGVSJLLH-UHFFFAOYSA-N
Application
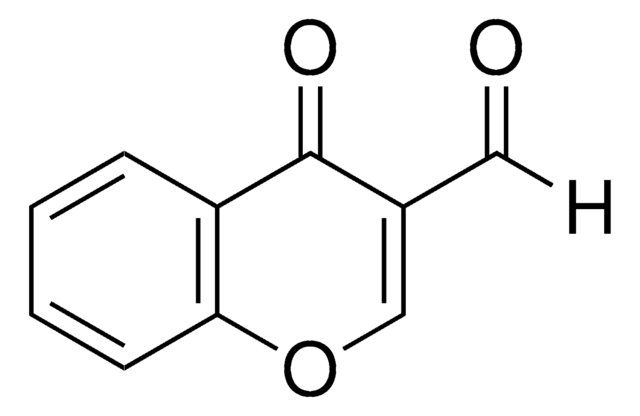
Methyl hydrazinocarboxylate (Methyl carbazate) was used in the preparation of methyl 3-(4-methyl-benzyl-idene)carbazate. It was also used in the synthesis of imidazo[1,5-d][1,2,4]triazines.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Yu-Feng Li et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 6), o1429-o1429 (2010-01-01)
The title compound, C(10)H(12)N(2)O(2), was prepared by the reaction of methyl carbazate and 4-methyl-benzaldehyde. The dihedral angle between the benzene ring and the carbazate fragment is 20.86 (10)°. In the crystal structure, mol-ecules are linked by inter-molecular N-H⋯O hydrogen bonds.
R Truhaut et al.
Toxicology, 22(3), 219-221 (1981-01-01)
Methyl carbazate, a metabolite of carbadox in the rat, was administered orally to rats for 2 years. The compound was mixed in the diet at concentrations corresponding to dose levels of 0, 2.5, 5 and 10 mg/kg body wt/day. There
R Paul et al.
Journal of medicinal chemistry, 28(11), 1704-1716 (1985-11-01)
By using inhibition of histamine release from antigen-challenged, sensitized human basophils as a means of identifying a potentially prophylactic drug for the treatment of asthma, a series of substituted imidazo[1,5-d][1,2,4]triazines were found, which were active. These compounds were prepared by
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico