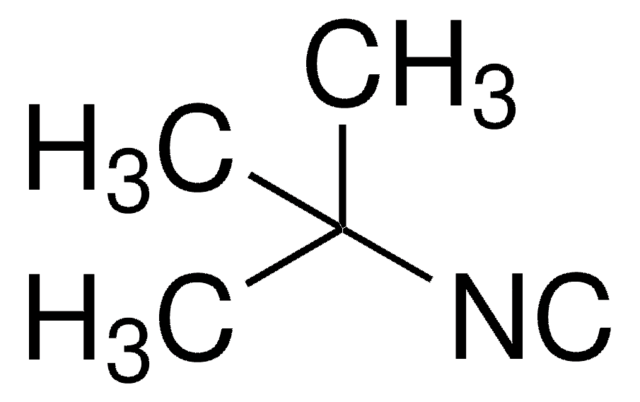
133299
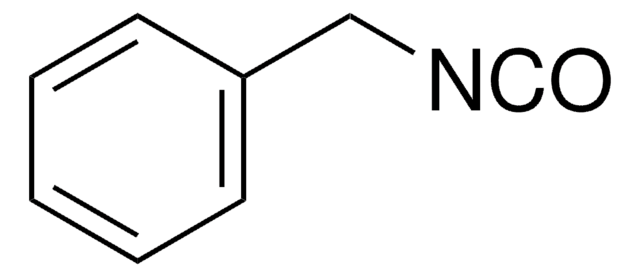
Benzyl isocyanide
98%
Sinónimos:
(Isocyanomethyl)benzene, Benzyl isonitrile
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
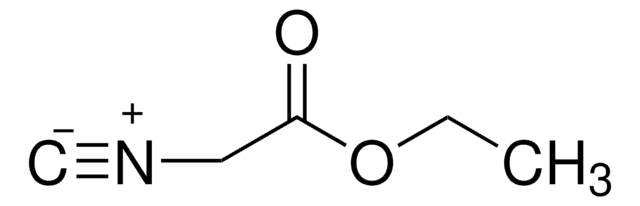
C6H5CH2NC
Número de CAS:
Peso molecular:
117.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
refractive index
n20/D 1.521 (lit.)
bp
105-106 °C/75 mmHg (lit.)
density
0.962 g/mL at 25 °C (lit.)
functional group
amine
storage temp.
−20°C
SMILES string
[C-]#[N+]Cc1ccccc1
InChI
1S/C8H7N/c1-9-7-8-5-3-2-4-6-8/h2-6H,7H2
InChI key
RIWNFZUWWRVGEU-UHFFFAOYSA-N
Categorías relacionadas
General description
Benzyl isocyanide forms phosphaalkene-containing complexes.
Application
Benzyl isocyanide was used in the synthesis of Ru(II) complexes containing hydrazine and benzyl isocyanide ligands. It was used in a three-component coupling process leading to O- and N-arylamides.
Other Notes
May darken in storage
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
174.2 °F - closed cup
flash_point_c
79 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Samantha N MacMillan et al.
Chemical communications (Cambridge, England), (40)(40), 4172-4174 (2007-10-11)
Reaction of (N(3)N)ZrPHPh (N(3)N=N(CH(2)CH(2)NSiMe(3))(3)(3-)) with PhCH(2)N[triple bond]C affords the 1,1-insertion product (N(3)N)Zr[C(PHPh)=NCH(2)Ph], which thermally rearranges to the phosphaalkene-containing complex, (N(3)N)Zr[N(CH(2)Ph)C(H)=PPh].
Laurent El Kaïm et al.
The Journal of organic chemistry, 72(11), 4169-4180 (2007-04-26)
The use of Smiles rearrangement in Ugi- and Passerini-type couplings with electron-deficient phenols allows very straightforward multicomponent formation of O-aryl- and N-arylamides. Best yields were observed with the highly activated o- and p-nitrophenols, salicylic derivatives giving adducts in lower yields.
Synthesis and X-ray studies of ruthenium (II) complexes containing hydrazine and benzyl isocyanide ligands.
Owalude SO, et al.
Bulletin of the Chemical Society of Ethiopia, 27(3), 405-411 (2013)
Zeinab Faghih et al.
Iranian journal of pharmaceutical research : IJPR, 19(3), 134-143 (2021-03-09)
The complex [(PhCH2NC)AuCl], 1, was prepared by the reaction of [(Me2S)AuCl], A, with an equimolar amount of benzyl isocyanide (PhCH2NC) ligand. Through a salt metathesis reaction, the chloride ligand in 1 was replaced by potassium benzothiazole-2-thiolate (Kbt) and potassium benzoimidazole-2-thiolate
Ben J Tickner et al.
Chemical science, 10(20), 5235-5245 (2019-06-14)
We report the formation of a series of novel [Ir(H)2(IMes)(α-13C2-carboxyimine)L] complexes in which the identity of the coligand L is varied. When examined with para-hydrogen, complexes in which L is benzylamine or phenethylamine show significant 1H hydride and 13C2 imine
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico