129593
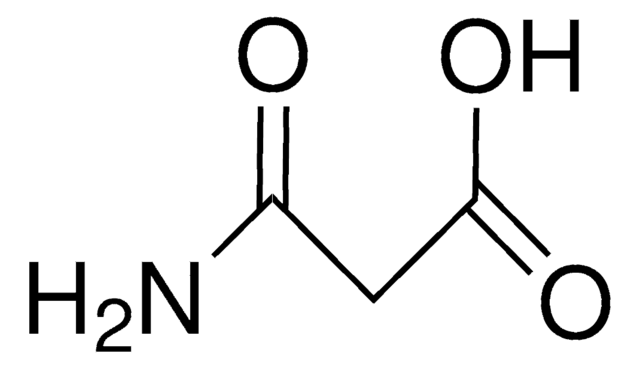
Malonamide
97%
Sinónimos:
Malonodiamide
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
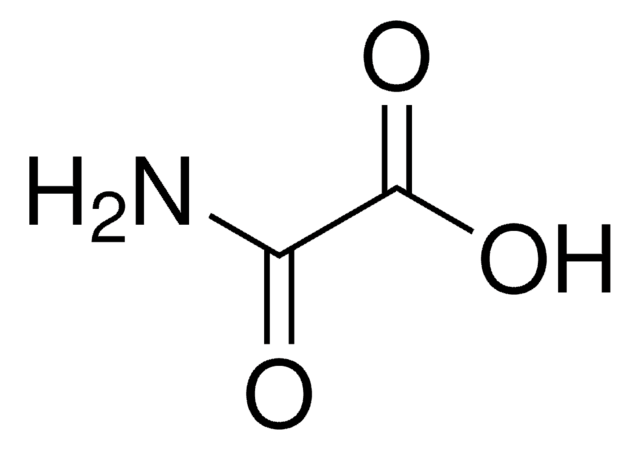
Fórmula lineal:
CH2(CONH2)2
Número de CAS:
Peso molecular:
102.09
Beilstein/REAXYS Number:
1751401
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
solid
mp
172-175 °C (lit.)
fluorescence
λex 367 nm; λem 445 nm (α-keto acid adduct)
SMILES string
NC(=O)CC(N)=O
InChI
1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7)
InChI key
WRIRWRKPLXCTFD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2.
Application
The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare-earth ions from nitric acid medium.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Alok Rout et al.
Dalton transactions (Cambridge, England : 2003), 43(4), 1862-1872 (2013-11-22)
A new non-fluorinated malonamide-based ionic liquid extractant was synthesized and investigated for the extraction behavior of europium(III) and other trivalent rare-earth ions from nitric acid medium. The extractant was the functionalized ionic liquid trihexyl(tetradecyl)phosphonium N,N,N',N'-tetra(2-ethylhexyl)malonate, [P66614][MA], and it was used
Ranjeet A Dhokale et al.
Organic letters, 14(15), 3994-3997 (2012-07-27)
A facile, fluoride-induced transition-metal-free chemoselective α-arylation of β-dicarbonyl compounds (malonamide esters) at room temperature using aryne intermediates has been demonstrated. Selective mono- or diarylation and generation of a quaternary benzylic stereocenter have also been achieved. The methodology will be highly
Suban K Sahoo et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 63(3), 574-586 (2005-07-19)
A new dipodal ligand, N,N'-bis{2-[(2-hydroxybenzylidine)amino]ethyl}malonamide (BHAEM) was synthesized by Schiff base condensation of N,N'-bis(2-aminoethyl)malonamide with two equivalent of salicylaldehyde and characterized on the basis of elemental analyses and various spectral (UV-vis, IR, (1)H NMR and (13)C NMR) data. The complexation
Amélie Banc et al.
The journal of physical chemistry. B, 115(6), 1376-1384 (2011-01-22)
In this paper we used a surfactant-stabilized lyotropic lamellar model system to study the interfacial behavior of an ion-extracting agent: N(1),N(3) dimethyl-N(1),N(3)-dibutyl-2-tetradecylmalonamide (DMDBTDMA). An analysis of small-angle X-ray scattering (SAXS) and polarized attenuated total reflectance-Fourier transform infrared (ATR-FTIR) data enabled
Mi-Hyun Kim et al.
Organic letters, 12(12), 2826-2829 (2010-05-27)
A new enantioselective synthetic method of (-)-paroxetine is reported. (-)-Paroxetine could be obtained in 15 steps (95% ee and 9.1% overall yield) from N,N-bis(p-methoxyphenyl)malonamide tert-butyl ester via the enantioselective phase-transfer catalytic alkylation and the diastereoselective Michael addition as the key
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico