122440
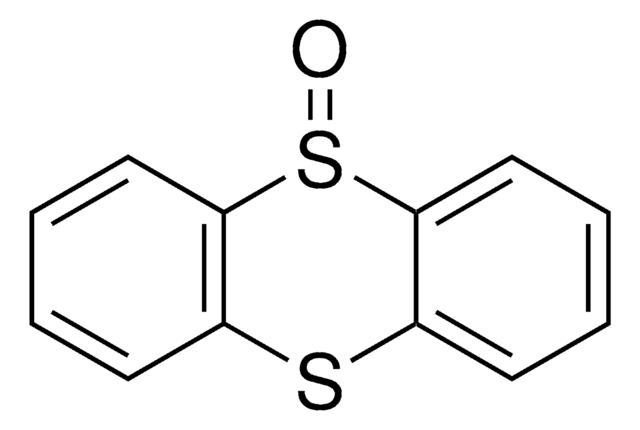
Thianthrene
97%
Sinónimos:
Dibenzodithiodioxane
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C12H8S2
Número de CAS:
Peso molecular:
216.32
Beilstein/REAXYS Number:
83046
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
solid
bp
364-366 °C (lit.)
mp
151-155 °C (lit.)
solubility
DMF: soluble
H2O: insoluble
SMILES string
S1c2ccccc2Sc3ccccc13
InChI
1S/C12H8S2/c1-2-6-10-9(5-1)13-11-7-3-4-8-12(11)14-10/h1-8H
InChI key
GVIJJXMXTUZIOD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Thianthrene undergoes liquid phase tert-butylation in the presence of large pore zeolites and mesoporous aluminosilicates catalyst to yield tert-butyl derivatives. Thianthrene on oxidation in the presence of hydrogen peroxide and ligninase as catalyst from Phanerochaete chrysosporium yields thianthrene monosulfoxide.
Application
Thianthrene has been used to study aqueous solubilities of several solid nitrogen-, sulfur- and oxygen-containing heterocyclic derivatives of anthracene, phenanthrene and fluorene. It has been used in determination of partition coefficients of several sulfur-containing aromatics in 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and supercritical carbon dioxide.
Related product
Referencia del producto
Descripción
Precios
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Pavel Karásek et al.
Journal of chromatography. A, 1140(1-2), 195-204 (2006-12-05)
We report the aqueous solubilities of phenanthrene and several solid three-ring aromatic heterocycles (phenanthridine, acridine, phenazine, thianthrene, phenothiazine, phenoxathiin, phenoxazine, carbazole, dibenzofuran, dibenzothiophene, and 4,6-dimethyldibenzothiophene) at temperatures ranging from 313K to the solute melting point and at a pressure of
Acid zeolites as catalysts in organic reactions.< i> tert</i>-Butylation of anthracene, naphthalene and thianthrene.
Armengol E, et al.
Applied Catalysis A: General, 149(2), 411-423 (1997)
Distribution of sulfur-containing aromatics between [hmim][Tf2N] and supercritical CO2: a case study for deep desulfurization of oil refinery streams by extraction with ionic liquids.
Planeta J, et al.
Green Chemistry, 8(1), 70-77 (2006)
R P Schreiner et al.
Applied and environmental microbiology, 54(7), 1858-1860 (1988-07-01)
The oxidation of heterocyclic sulfur compounds reported to be part of the macrostructure of coal and petroleum was investigated. The oxidation of thianthrene solubilized in 10% dimethylformamide to thianthrene monosulfoxide in the presence of hydrogen peroxide was catalyzed by the
Saowapak Kaenkaew et al.
Journal of molecular modeling, 16(2), 243-253 (2009-07-10)
The structures of thiacalix[2]thianthrene, p-tert-butylthiacalix[2]thianthrene and their complexes with Zn(2+), Cd(2+) and Hg(2+) were obtained using B3LYP/LanL2DZ and HF/LanL2DZ calculations. The structures of the most stable conformers of thiacalix[2]thianthrene and p-tert-butylthiacalix[2]thianthrene optimized at either the B3LYP/LanL2DZ or HF/LanL2DZ level are
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico