119792
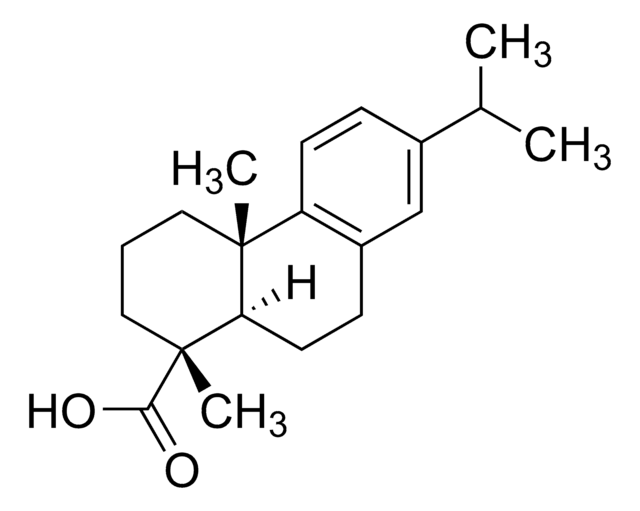
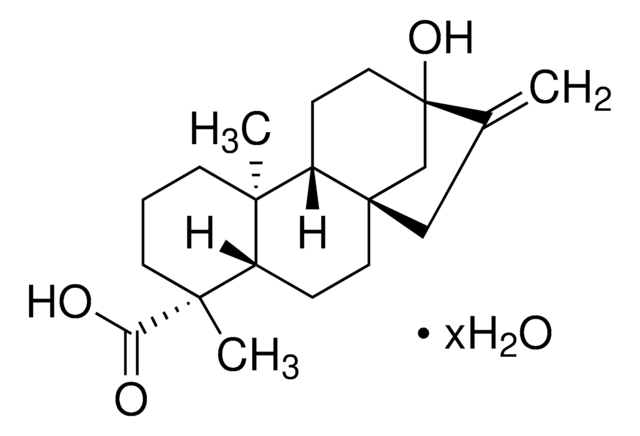
Podocarpic acid
98%
Sinónimos:
(+)-Podocarpic acid, (1S)-1,2,3,4,4a,9,10,10a-Octahydro-6-hydroxy-1,4a-dimethyl-1-phenanthrenecarboxylic acid, (1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-Octahydro-6-hydroxy-1,4a-dimethyl-1-phenanthrenecarboxylic acid, Podocarpic acid (resin acid)
About This Item
Productos recomendados
Quality Level
assay
98%
form
solid
optical activity
[α]20/D +133°, c = 4 in ethanol
mp
193-196 °C (lit.)
functional group
carboxylic acid
SMILES string
[H][C@@]12CCc3ccc(O)cc3[C@@]1(C)CCC[C@]2(C)C(O)=O
InChI
1S/C17H22O3/c1-16-8-3-9-17(2,15(19)20)14(16)7-5-11-4-6-12(18)10-13(11)16/h4,6,10,14,18H,3,5,7-9H2,1-2H3,(H,19,20)/t14-,16-,17+/m1/s1
InChI key
VJILEYKNALCDDV-OIISXLGYSA-N
Gene Information
human ... TNF(7124)
Categorías relacionadas
Application
- (+)-Podocarpic acid as chiral template in the synthesis of aphidicolane, stemodane and stemarane diterpenoids: This article reviews the use of (+)-podocarpic acid in the synthesis of various diterpenoids, showcasing its utility in complex organic syntheses (La Bella et al., 2016).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico