103713
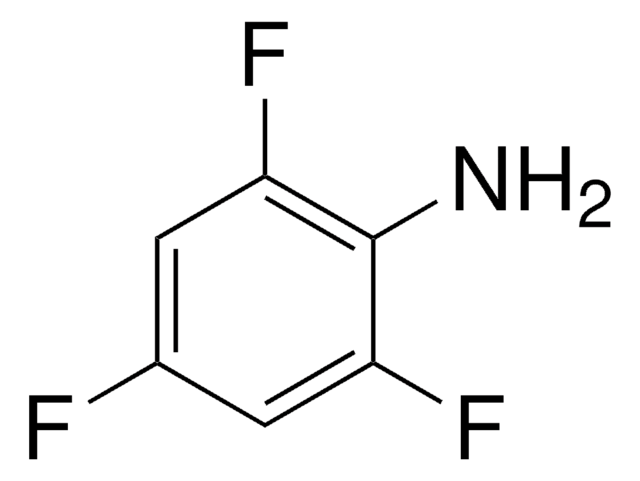
2,3,4,5,6-Pentafluoroaniline
99%
Sinónimos:
Pentafluoroaniline
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6F5NH2
Número de CAS:
Peso molecular:
183.08
Beilstein/REAXYS Number:
1819387
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
solid
bp
153 °C (lit.)
mp
33-35 °C (lit.)
solubility
toluene: soluble
functional group
fluoro
SMILES string
Nc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6H2F5N/c7-1-2(8)4(10)6(12)5(11)3(1)9/h12H2
InChI key
NOXLGCOSAFGMDV-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
2,3,4,5,6-Pentafluoroaniline forms metal-drug complexes, cis-Pt-(2,3,4,5,6-pentafluoroaniline)2-Br2 which has been tested against the promastigote forms of Leishmania donovani.
Application
2,3,4,5,6-Pentafluoroaniline may be used in the preparation of pentafluorophenylammonium triflate, an efficient catalyst for esterification and thioesterification. 2,3,4,5,6-Pentafluoroaniline was used in synthesis of various titanium complexes having two anionic [N, O–] bidentate salicylaldiminato ligands.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
163.4 °F
flash_point_c
73 °C
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
C M Mesa-Valle et al.
The Journal of antimicrobial chemotherapy, 40(1), 47-57 (1997-07-01)
The action of 16 newly synthesized metal complexes having the general structure cis-Pt-(II)-Xn-Ln have been tested in vitro against the promastigote forms of Leishmania donovani. The metal complexes at 24 h and maximum dosages inhibited growth from 0%, e.g. in
Bis (salicylaldiminato) titanium complexes containing bulky imine substituents: Synthesis, characterization and ethene polymerization studies.
Parssinen A, et al.
European Journal of Inorganic Chemistry, 2005(11), 2100-2109 (2005)
Pentafluorophenylammonium triflate (PFPAT): an efficient, practical, and cost-effective catalyst for esterification, thioesterification, transesterification, and macrolactone formation.
Funatomi T, et al.
Green Chemistry, 8(12), 1022-1027 (2006)
Vasily A Ilichev et al.
Dalton transactions (Cambridge, England : 2003), 48(3), 1060-1066 (2019-01-03)
To obtain new efficient lanthanide-based NIR luminophores perfluorinated 2-mercaptobenzothiazole was used as a ligand. The ate-complexes [(Ln(mbtF)4)-(Na(DME)3)+] of Nd (1), Sm (2), Tb (3), Er (4), Yb (5) and [(Y(mbtF)4)-(Li(DME)3)+] (6) were synthesized in high yields by the reactions of
I M Rietjens et al.
Chemico-biological interactions, 77(3), 263-281 (1991-01-01)
Metabolism and bioactivation of fluoroanilines was studied both in vitro in microsomal systems and in vivo. 4-Fluoroaniline and pentafluoroaniline and their non-para fluorinated analogues were used as the model compounds. Special attention was focussed on bioactivation to reactive benzoquinoneimines. Cytochrome
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| 103713-100G | |
| 103713-25G | 4061838670724 |
| 103713-5G | 4061838670731 |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico