P3418
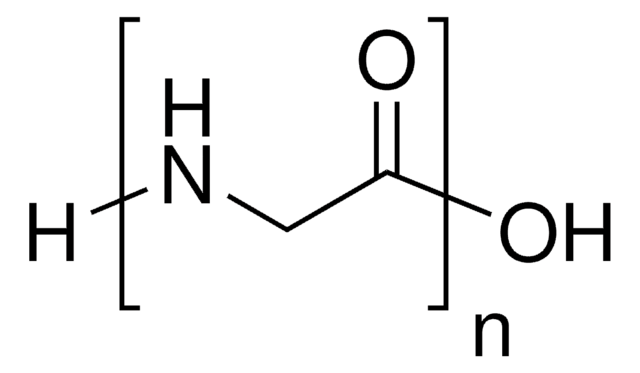
Poly-(α,β)-DL-aspartic acid sodium salt
mol wt 2,000-11,000
Synonym(s):
Aspartic acid homopolymer sodium salt
About This Item
Recommended Products
Looking for similar products? Visit Product Comparison Guide
Application
- Improving the effectiveness of (-)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid.: This research highlights the development of nanoparticles using polyaspartic acid to enhance the bioavailability and therapeutic effect of EGCG in treating atherosclerosis (Hong et al., 2014).
- Polyaspartate, a biodegradable chelant that improves the phytoremediation potential of poplar in a highly metal-contaminated agricultural soil.: The study evaluates the use of polyaspartate in enhancing the phytoremediation capabilities of poplar trees, showcasing its environmental application for soil decontamination (Lingua et al., 2014).
- Modulation of calcium oxalate dihydrate growth by selective crystal-face binding of phosphorylated osteopontin and polyaspartate peptide showing occlusion by sectoral (compositional) zoning.: This research investigates the role of polyaspartate in modulating the growth of calcium oxalate crystals, with implications for understanding kidney stone formation (Chien et al., 2009).
Preparation Note
Analysis Note
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Humankind has utilized protein materials throughout its existence, starting with the use of materials such as wool and silk for warmth and protection from the elements and continuing with the use of recombinant DNA techniques to synthesize proteins with unique and useful properties.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service