E2271
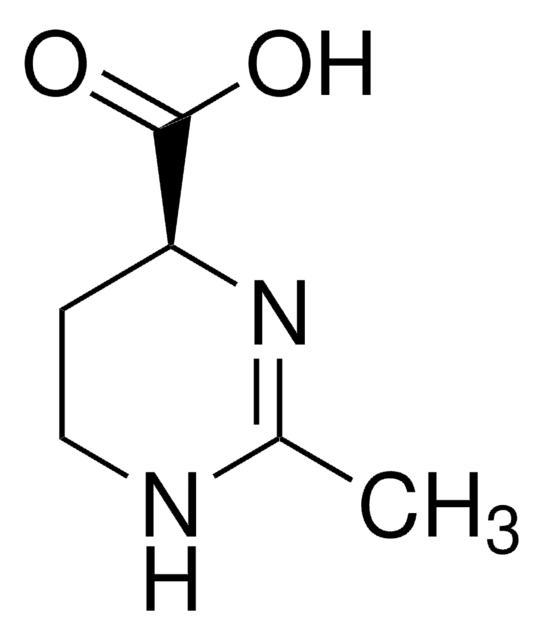
Ectoine
osmoprotectant
Synonym(s):
(S)-2-Methyl-1,4,5,6-tetrahydropyrimidine-4-carboxylic acid, Thp(B)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H10N2O2
CAS Number:
Molecular Weight:
142.16
Beilstein:
7288977
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
≥98.0% (TLC)
form
powder
mol wt
142.16
storage temp.
room temp
SMILES string
CC1=N[C@@H](CCN1)C(O)=O
InChI
1S/C6H10N2O2/c1-4-7-3-2-5(8-4)6(9)10/h5H,2-3H2,1H3,(H,7,8)(H,9,10)/t5-/m0/s1
InChI key
WQXNXVUDBPYKBA-YFKPBYRVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ectoine is a natural cyclic tetrahydropyrimidine, which is a low molecular weight, water-binding and organic osmolyte. It is a part of a class of small molecule chaperones (SMCs).
Biochem/physiol Actions
Ectoine acts as an osmoprotectant in a wide variety of microorganisms including heterotrophic, halophilic bacteria and non-halophilic bacteria such as Streptomyces species and E.coli. This compatible solute exhibit protective effects in E.coli during drying and storage. Ectoine is implicated in protein and lipid bilayer stabilization. It also regulates the preservation of turgor pressure and ameliorates desiccation stress. It may be used to treat inflammatory diseases such as, allergic rhinitis, atopic dermatitis and chronic obstructive pulmonary disease. Ectoine can cause DNA structural changes in vitro and protects DNA from ionizing radiation (IR). It prevents insulin amyloid formation in vitro.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer?s beta-amyloid
Kanapathipillai M, et al.
Febs Letters, 579(21), 4775-4780 (2005)
Jens Smiatek et al.
Biophysical chemistry, 160(1), 62-68 (2011-10-22)
We have performed Molecular Dynamics simulations of ectoine, hydroxyectoine and urea in explicit solvent. Special attention has been spent on the local surrounding structure of water molecules. Our results indicate that ectoine and hydroxyectoine are able to accumulate more water
Ruediger Graf et al.
Clinics in dermatology, 26(4), 326-333 (2008-08-12)
The protective properties of ectoine, formerly described for only extremophilic microorganisms, can be transferred to human skin. Our present data show that the compatible solute ectoine protects the cellular membrane from damage caused by surfactants. Transepidermal water loss measurements in
Ralf Spindler et al.
Cryobiology, 64(3), 250-260 (2012-02-22)
Cellular response during the freeze-thaw process strongly affects the cryopreservation outcome including cell morphology and cell viability. Cryomicroscopy was used to individually analyze the osmotic response of human pulmonary microvascular endothelial cells (HPMECs) during slow cooling (1 °C/min) to -60
Naomi Ofer et al.
Applied and environmental microbiology, 78(20), 7483-7486 (2012-08-14)
Mycobacterium smegmatis is a commonly used mycobacterial model system. Here, we show that M. smegmatis protects itself against elevated salinity by synthesizing ectoine and hydroxyectoine and characterize the phenotype of a nonproducing mutant. This is the first analysis of M.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service