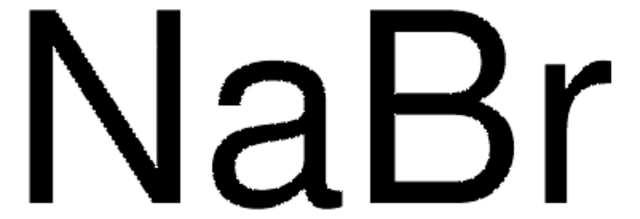746401
Sodium bromide
anhydrous, free-flowing, Redi-Dri™, ReagentPlus®, ≥99%
Sign Into View Organizational & Contract Pricing
All Photos(7)
About This Item
Linear Formula:
NaBr
CAS Number:
Molecular Weight:
102.89
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.21
Assay:
≥99%
grade:
anhydrous
form:
powder
Recommended Products
grade
anhydrous
Quality Level
vapor pressure
1 mmHg ( 806 °C)
product line
ReagentPlus®
Redi-Dri™
Assay
≥99%
form
powder
quality
free-flowing
pH
5.4 (20 °C, 50 g/L)
mp
755 °C (lit.)
SMILES string
[Na+].[Br-]
InChI
1S/BrH.Na/h1H;/q;+1/p-1
InChI key
JHJLBTNAGRQEKS-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Co-catalyst for TEMPO/NaOCl oxidation of alcohols in non-chlorinated solvents.
Towards greener solvents for the bleach oxidation of alcohols catalysed by stable N-oxy radicals
Towards greener solvents for the bleach oxidation of alcohols catalysed by stable N-oxy radicals
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Redi-Dri is a trademark of Sigma-Aldrich Co. LLC
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2 - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Patricia Cazón et al.
Carbohydrate polymers, 216, 72-85 (2019-05-03)
Bacterial cellulose (BC) produced by Komagataeibacter xylinus is a biomaterial with a unique three-dimensional structure. To improve the mechanical properties and reinforce the BC films, they were immersed in polyvinyl alcohol (0-4%) and chitosan (0-1%) baths. Moisture content, mechanical properties
Patricia Cazón et al.
International journal of biological macromolecules, 117, 235-246 (2018-05-25)
The aim of this study was to develop composite films from cellulose, chitosan and polyvinyl alcohol to obtain environmentally friendly materials. Toughness, burst strength, distance to burst and water adsorption properties were measured and analysed as a function of cellulose
Christian Freese et al.
Particle and fibre toxicology, 11, 68-68 (2014-12-30)
In general the prediction of the toxicity and therapeutic efficacy of engineered nanoparticles in humans is initially determined using in vitro static cell culture assays. However, such test systems may not be sufficient for testing nanoparticles intended for intravenous application.
Ramon Weishaupt et al.
Biomacromolecules, 16(11), 3640-3650 (2015-09-29)
Controlled and efficient immobilization of specific biomolecules is a key technology to introduce new, favorable functions to materials suitable for biomedical applications. Here, we describe an innovative and efficient, two-step methodology for the stable immobilization of various biomolecules, including small
Yi-Ling Hsieh et al.
Pharmaceutical research, 32(2), 549-561 (2014-08-26)
The aim of this study was to investigate how factors such as temperature, relative humidity and particle size impact the extent of disproportionation (salt to free base conversion) in powder blends of miconazole, benzocaine or sertraline mesylate salts mixed with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service