379999
Ammonium carbonate
99.999% trace metals basis
Synonym(s):
Hartshorn salt
About This Item
Recommended Products
grade
for inorganic trace analysis
vapor density
2.7 (vs air)
vapor pressure
1,013.25 hPa °F
description
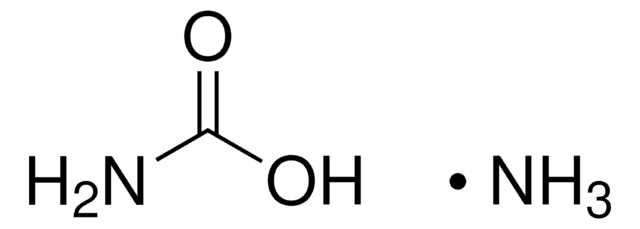
mixture of variable proportions of ammonium bicarbonate and ammonium carbamate
Assay
99.999% trace metals basis
form
powder
solubility
soluble (Water: slightly soluble)
SMILES string
N.N.OC(O)=O
InChI
1S/CH2O3.2H3N/c2-1(3)4;;/h(H2,2,3,4);2*1H3
InChI key
PRKQVKDSMLBJBJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a dispersant for SiO2 nano particles for dynamic light scattering, Z-potential, and centrifugal liquid sedimentation measurements.
- As an eluent in the separation of silica nanoparticles by asymmetric flow field-flow fractionation (AF4) coupled to inductively coupled plasma mass spectrometry (ICPMS) method.
- In the synthesis of ZnO nanoparticles.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
LC/MS Analysis of Glyphosate and Metabolites on apHera™ NH2, 2 mm I.D. Column
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service