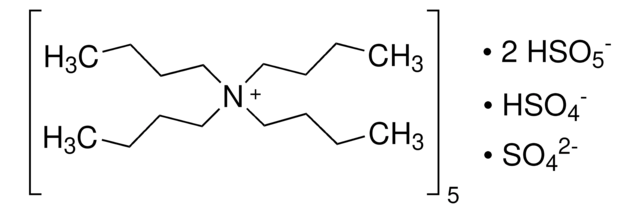
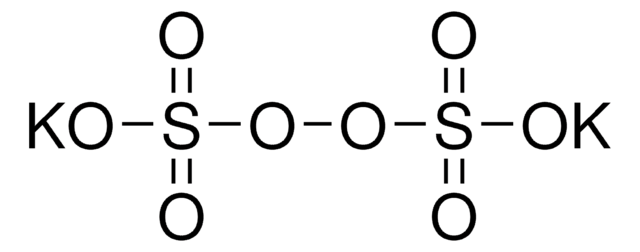228036
OXONE®, monopersulfate compound
Synonym(s):
Potassium peroxymonosulfate
About This Item
Recommended Products
vapor pressure
<0.0000017 hPa
Quality Level
form
powder
reaction suitability
reagent type: oxidant
concentration
>4.0% (active oxygen basis (by Na2S2O3, titration))
pH
2.1 (77 °C, 30 g/L)
SMILES string
[K+].[K+].[K+].[K+].[K+].OS([O-])(=O)=O.[O-]S([O-])(=O)=O.O[S+]([O-])([O-])(=O)=O.O[S+]([O-])([O-])(=O)=O
InChI
1S/5K.2H2O5S.2H2O4S/c;;;;;2*1-5-6(2,3)4;2*1-5(2,3)4/h;;;;;2*1H,(H,2,3,4);2*(H2,1,2,3,4)/q5*+1;;;;/p-5
InChI key
HJKYXKSLRZKNSI-UHFFFAOYSA-I
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
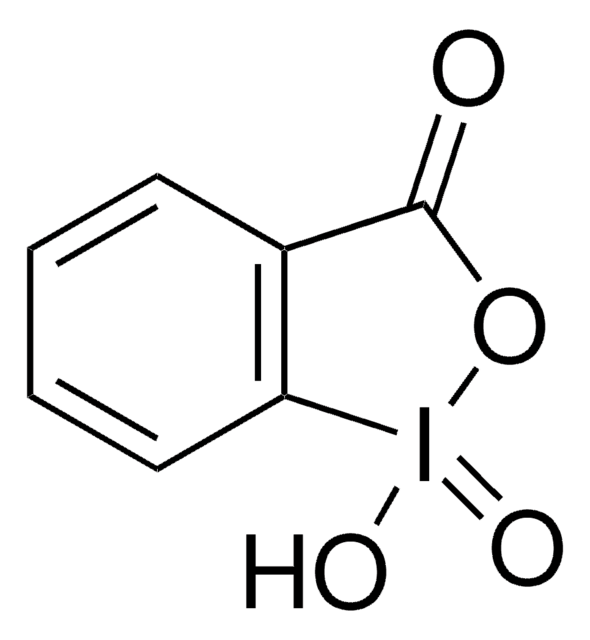
2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone
A Convenient Halogenation of α,β-Unsaturated Carbonyl Compounds with OXONE® and Hydrohalic Acid (HBr, HCl)
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service