About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥95%
refractive index
n20/D 1.445 (lit.)
bp
240 °C (lit.)
density
0.891 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
citronellol, geraniol
Organoleptic
green; citrus; fruity; floral; rose
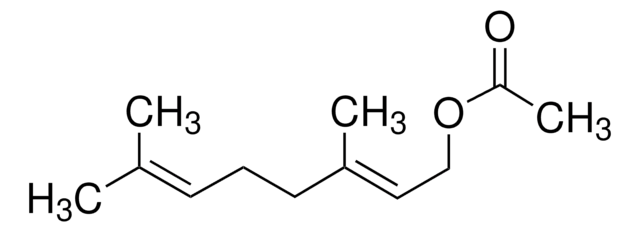
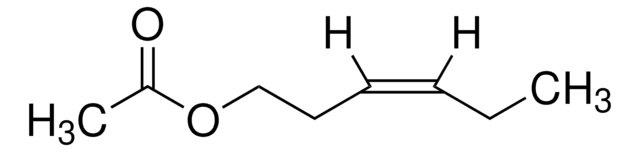
SMILES string
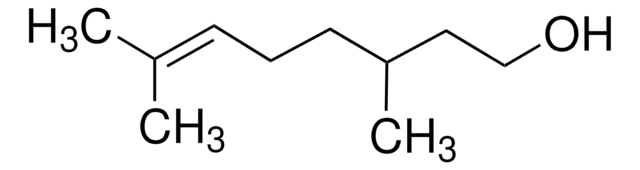
CC(CCOC(C)=O)CC\C=C(\C)C
InChI
1S/C12H22O2/c1-10(2)6-5-7-11(3)8-9-14-12(4)13/h6,11H,5,7-9H2,1-4H3
InChI key
JOZKFWLRHCDGJA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
217.4 °F - closed cup
Flash Point(C)
103 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service