B12385
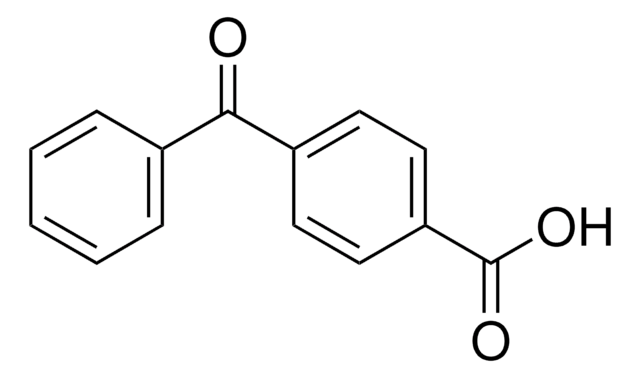
2-Benzoylbenzoic acid
98%
Synonym(s):
Benzophenone-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5COC6H4CO2H
CAS Number:
Molecular Weight:
226.23
Beilstein:
1107841
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
257-265 °C (lit.)
mp
126-129 °C (lit.)
SMILES string
OC(=O)c1ccccc1C(=O)c2ccccc2
InChI
1S/C14H10O3/c15-13(10-6-2-1-3-7-10)11-8-4-5-9-12(11)14(16)17/h1-9H,(H,16,17)
InChI key
FGTYTUFKXYPTML-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiao Wang et al.
Organic letters, 13(4), 709-711 (2011-01-15)
2-Benzoylbenzoyl azides undergo facile cyclization under acidic conditions to give substituted dibenzo[b,f][1,5]-diazocines in good yields. This approach shortens the synthetic steps toward these compounds as compared with conventional methods. The mechanism of the diazocine synthesis is assumed to proceed by
Quentin Duez et al.
Polymers, 11(4) (2019-04-19)
Several families of polymers possessing various end-groups are characterized by ion mobility mass spectrometry (IMMS). A significant contribution of the end-groups to the ion collision cross section (CCS) is observed, although their role is neglected in current fitting models described
Marianne Placzek et al.
Acta dermato-venereologica, 93(1), 30-32 (2012-09-18)
Benzophenone is a phototoxic compound with absorption maxima in the ultraviolet A (UVA) and ultraviolet B (UVB) range. Many benzophenone derivatives are known to be photosensitizing. On the other hand, 2-hydroxy-4-methoxybenzophenone is used as a photoprotective agent. The aim of
Stanislav Gobec et al.
Bioorganic & medicinal chemistry letters, 15(23), 5170-5175 (2005-09-27)
Nonsteroidal anti-inflammatory drugs (NSAIDs) like indomethacin, flufenamic acid, and related compounds have been recently identified as potent inhibitors of AKR1C3. We report that some other NSAIDs (diclofenac and naproxen) also inhibit AKR1C3, with the IC(50) values in the low micromolar
Wojciech Ostrowski et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 967, 21-27 (2014-07-27)
Liquid chromatography coupled to mass spectrometry (MS) with electrospray ionization (ESI) is one of analytical techniques to obtain accurate results of low molecular weight aromatic compounds in biological samples of different origin. The interpretations of mass spectra of these aromatic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service