A68203
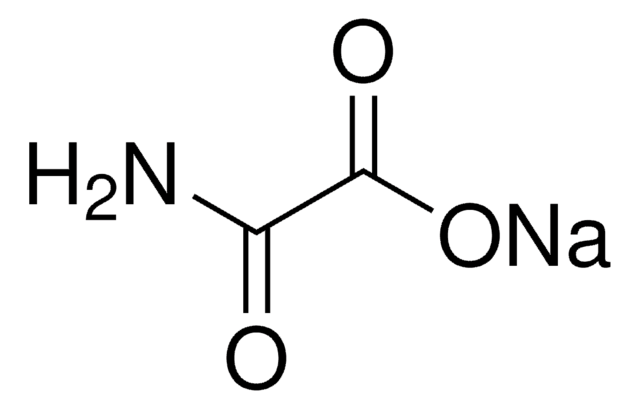
6-Aminonicotinamide
99%
Synonym(s):
6AN, 6-Aminopyridine-3-carboxamide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H7N3O
CAS Number:
Molecular Weight:
137.14
Beilstein:
116042
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
245-248 °C (lit.)
SMILES string
NC(=O)c1ccc(N)nc1
InChI
1S/C6H7N3O/c7-5-2-1-4(3-9-5)6(8)10/h1-3H,(H2,7,9)(H2,8,10)
InChI key
ZLWYEPMDOUQDBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Aminonicotinamide is an aminopyridine, which is a specific pentose inhibitor and thus inhibits the NADP production.
Application
6-Aminonicotinamide can be used as a reactant:
- For the synthesis of 6-substituted imidazo[1,2-a]pyridines with potential application as chemotherapeutic drugs.
- In the dehydrative N-monobenzylation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A borrowing hydrogen methodology: palladium-catalyzed dehydrative N-benzylation of 2-aminopyridines in water.
Hikawa H, et al.
Green Chemistry, 20(13), 3044-3049 (2018)
6-Substituted imidazo [1, 2-a] pyridines: Synthesis and biological activity against colon cancer cell lines HT-29 and Caco-2.
Dahan-Farkas N, et al.
European Journal of Medicinal Chemistry, 46(9), 4573-4583 (2011)
Laurent M Paardekooper et al.
Frontiers in immunology, 10, 1216-1216 (2019-06-14)
Neutrophils kill ingested pathogens by the so-called oxidative burst, where reactive oxygen species (ROS) are produced in the lumen of phagosomes at very high rates (mM/s), although these rates can only be maintained for a short period (minutes). In contrast
Electrocatalytic synthesis of 6-aminonicotinic acid at silver cathodes under mild conditions.
Gennaro A, et al.
Electrochemical Communications, 6(7), 627-631 (2004)
Jorge Domínguez-Andrés et al.
PLoS pathogens, 13(9), e1006632-e1006632 (2017-09-19)
Monocytes are innate immune cells that play a pivotal role in antifungal immunity, but little is known regarding the cellular metabolic events that regulate their function during infection. Using complementary transcriptomic and immunological studies in human primary monocytes, we show
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service