86734
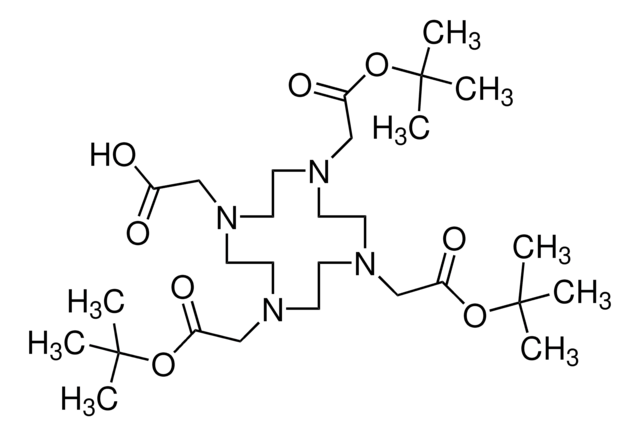
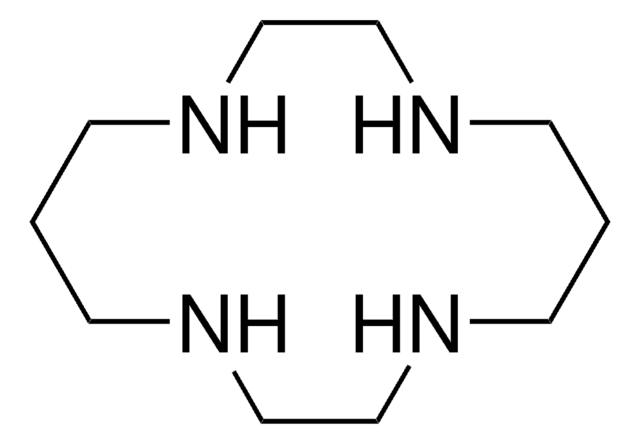
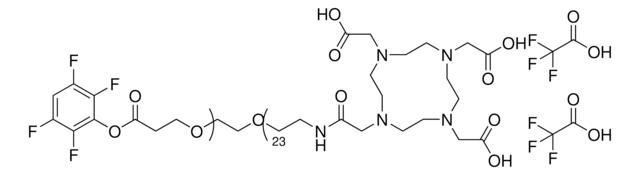
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
≥97.0% (CHN)
Synonym(s):
DOTA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H28N4O8 · xH2O
CAS Number:
Molecular Weight:
404.42 (anhydrous basis)
Beilstein:
1186987
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (CHN)
form
powder
SMILES string
OC(=O)CN1CCN(CCN(CCN(CC1)CC(O)=O)CC(O)=O)CC(O)=O
InChI
1S/C16H28N4O8/c21-13(22)9-17-1-2-18(10-14(23)24)5-6-20(12-16(27)28)8-7-19(4-3-17)11-15(25)26/h1-12H2,(H,21,22)(H,23,24)(H,25,26)(H,27,28)
InChI key
WDLRUFUQRNWCPK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) is a macrocyclic complexing agent.
- It has been used for radiolabeling of carbon nanotube bioconjugates by chelating 64Cu radioisotope.
- DOTA can be activated with N-hydroxysulfosuccinimidyl for conjugation with monoclonal antibodies in clinical radioimmunotherapy.
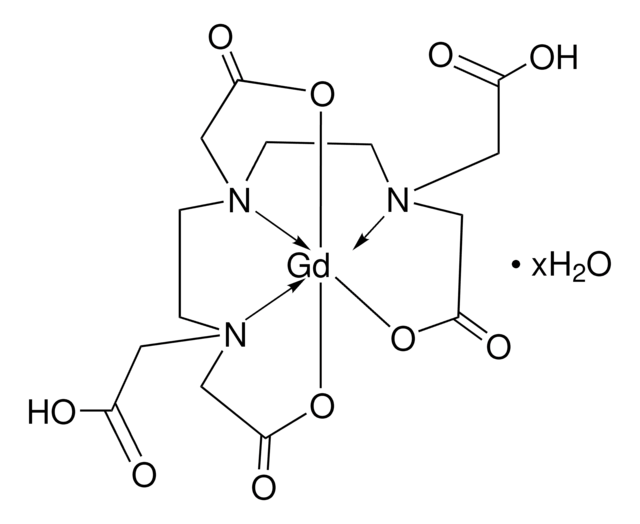
- It can form complexes with gadolinium for application as MRI contrast agents.
- Metal complexes of DOTA-peptide conjugates can be used as therapeutic radiopharmaceuticals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jack J Miller et al.
NMR in biomedicine, 31(6), e3912-e3912 (2018-04-11)
The aim of this work was to investigate the use of 13 C-labelled acetoacetate and β-hydroxybutyrate as novel hyperpolarized substrates in the study of cardiac metabolism. [1-13 C]Acetoacetate was synthesized by catalysed hydrolysis, and both it and [1-13 C]β-hydroxybutyrate were
Javad Garousi et al.
Scientific reports, 7(1), 5961-5961 (2017-07-22)
Several anti-cancer therapies target the epidermal growth factor receptor (EGFR). Radionuclide imaging of EGFR expression in tumours may aid in selection of optimal cancer therapy. The
B Klaan et al.
Der Radiologe, 59(8), 710-721 (2019-07-10)
The imaging of chondral pathologies is an essential part in the work-up of acute and chronic joint diseases. Besides conventional MR imaging, CT and MR arthrography are well-established methods in evaluating articular cartilage. The application of these techniques requires knowledge
Luca Garbuio et al.
The journal of physical chemistry. B, 117(11), 3145-3153 (2013-02-28)
We present the first example of chemoselective site-specific spin labeling of a monomeric protein with two spectroscopically orthogonal spin labels: a gadolinium(III) chelate complex and a nitroxide radical. A detailed analysis of the performance of two commercially available Gd(III) ligands
Anna Orlova et al.
European journal of nuclear medicine and molecular imaging, 40(3), 439-449 (2012-11-28)
Radionuclide imaging of insulin-like growth factor type 1 receptor (IGF-1R) expression in tumours might be used for selection of patients who would benefit from IGF-1R-targeted therapy. We have previously shown the feasibility of IGF-1R imaging using the Affibody molecule (111)In-DOTA-His(6)-Z(IGF1R:4551).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service