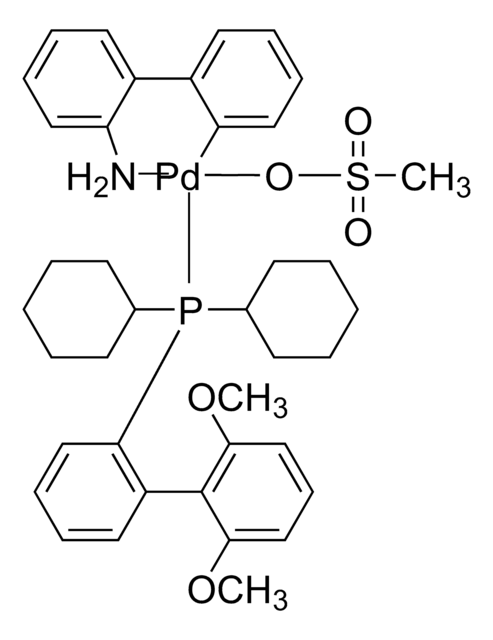
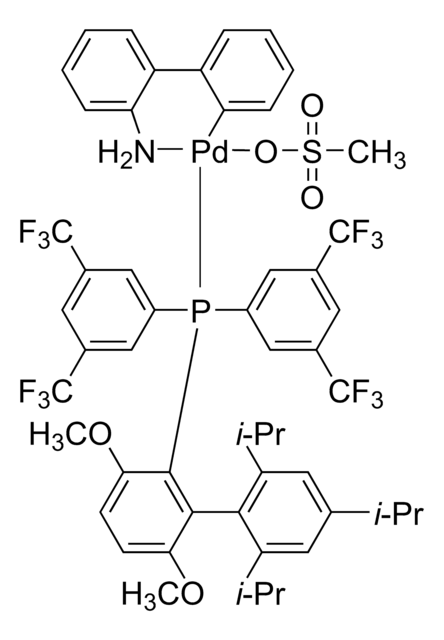
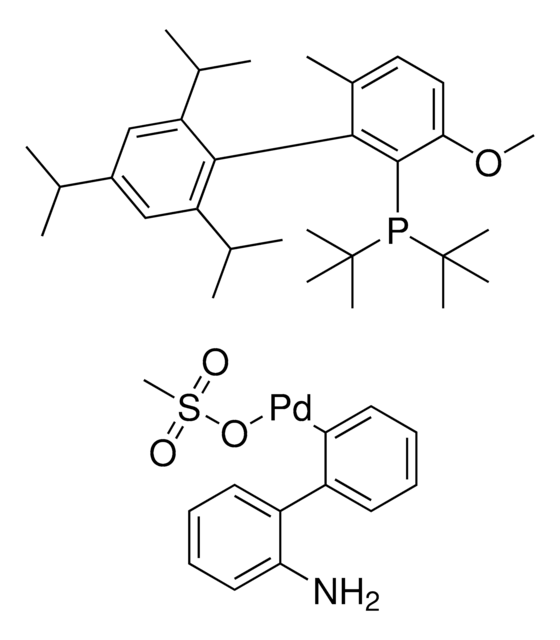
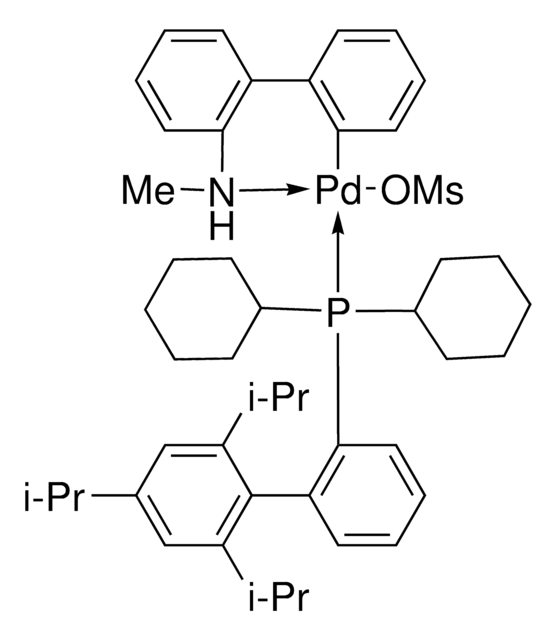
761435
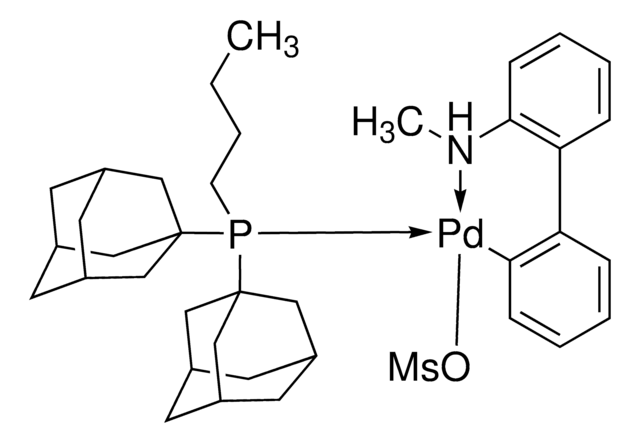
cataCXium® A Pd G3
95%
Synonym(s):
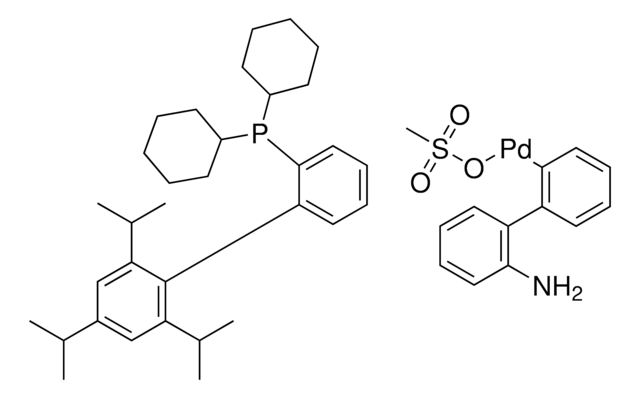
Mesylate[(di(1-adamantyl)-n-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II), [(Di(1-adamantyl)-butylphosphine)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate, cataCXium-A-Pd-G3
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
feature
generation 3
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
greener alternative product score
old score: 16
new score: 2
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Atom Economy
Safer Solvents and Auxiliaries
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤3% acetone
mp
196-241 °C (decomposition)
functional group
phosphine
greener alternative category
SMILES string
CS(=O)(=O)O[Pd]c1ccccc1-c2ccccc2N.CCCCP([C@@]34C[C@@H]5C[C@@H](C[C@@H](C5)C3)C4)[C@@]67C[C@@H]8C[C@@H](C[C@@H](C8)C6)C7
InChI
1S/C24H39P.C12H10N.CH4O3S.Pd/c1-2-3-4-25(23-11-17-5-18(12-23)7-19(6-17)13-23)24-14-20-8-21(15-24)10-22(9-20)16-24;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h17-22H,2-16H2,1H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1/t17-,18+,19-,20-,21+,22-,23-,24-;;;
InChI key
REYVZCOGMIXVNX-DVBMAMJVSA-M
General description
Application
- Direct ortho-arylation of pyridinecarboxylic acids.
- Catalyzing Suzuki–Miyaura cross-coupling in the synthesis of 1-heteroaryl-3-azabicyclo[3.1.0]hexanes.
- Palladium-catalyzed carbonylative carboperfluoroalkylation of alkynes.
- Suzuki–Miyaura coupling reaction of geminal bis(boryl)cyclopropanes in the synthesis of various gem-disubstituted cyclopropanes.
- Boroperfluoroalkylation of terminal alkynes.
- Copper-free Sonogashira coupling reaction of aromatic halides with alkynes to form C-C bond.
- Suzuki cross-coupling between organotrifluoroborate and aryl halides.
Legal Information
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Multiple tools have been created to ensure your success with kit set up. Start with the more detailed guide to ensure you are comfortable with all of the steps before using the quick guides on the excel worksheet. Remember that while the technique is new, it is still organic chemistry and so the steps will seem easy once you try just one kit. It is just a new way of approaching something you are already very good at.
Materials Included in your KITALYSIS-24PD-2PK High-Throughput Screening Kit
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)