754935
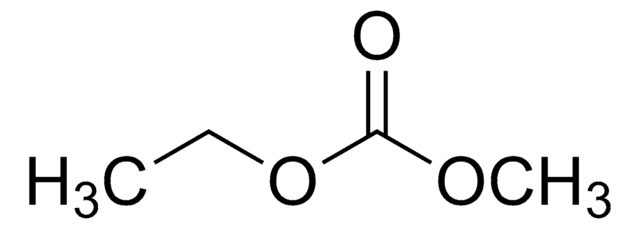
Ethyl methyl carbonate
99%
Synonym(s):
Carbonic acid ethyl methyl ester, EMC, Methyl ethyl carbonate
About This Item
Recommended Products
Quality Level
Assay
99%
form
liquid
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.378
bp
101 °C
mp
-55 °C
density
1.006 g/mL at 25 °C
application(s)
battery manufacturing
greener alternative category
, Enabling
SMILES string
CCOC(=O)OC
InChI
1S/C4H8O3/c1-3-7-4(5)6-2/h3H2,1-2H3
InChI key
JBTWLSYIZRCDFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
75.0 °F
Flash Point(C)
23.9 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service