721328
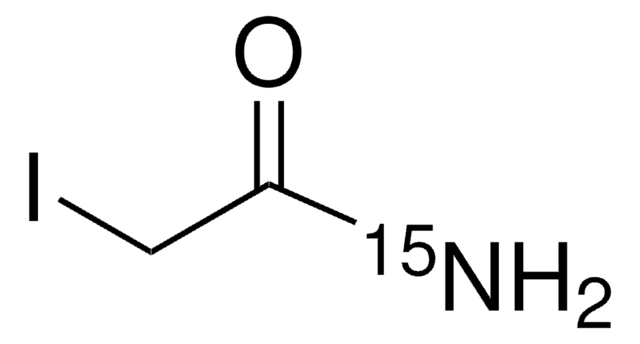
Iodoacetamide-13C2, 2-d2
99 atom % 13C, 98 atom % D
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
I13CD213CONH2
CAS Number:
Molecular Weight:
188.96
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.12
Recommended Products
isotopic purity
99 atom % 13C
98 atom % D
Quality Level
Assay
≥99% (GC)
form
powder
mp
92-95 °C (lit.)
mass shift
M+4
storage temp.
2-8°C
SMILES string
[2H][13C]([2H])(I)[13C](N)=O
InChI
1S/C2H4INO/c3-1-2(4)5/h1H2,(H2,4,5)/i1+1D2,2+1
InChI key
PGLTVOMIXTUURA-HDZHEIKLSA-N
Related Categories
General description
Iodoacetamide-13C2, isotopically labeled analogue of 2-Iodoacetamide.
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 4 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhonghua Wang et al.
Cell reports, 24(4), 815-823 (2018-07-26)
SAMHD1 is a dNTP triphosphohydrolase (dNTPase) that impairs retroviral replication in a subset of non-cycling immune cells. Here we show that SAMHD1 is a redox-sensitive enzyme and identify three redox-active cysteines within the protein: C341, C350, and C522. The three
Jessica N Spradlin et al.
Nature chemical biology, 15(7), 747-755 (2019-06-19)
Nimbolide, a terpenoid natural product derived from the Neem tree, impairs cancer pathogenicity; however, the direct targets and mechanisms by which nimbolide exerts its effects are poorly understood. Here, we used activity-based protein profiling (ABPP) chemoproteomic platforms to discover that
Charles A Berdan et al.
Cell chemical biology, 26(7), 1027-1035 (2019-05-14)
Parthenolide, a natural product from the feverfew plant and member of the large family of sesquiterpene lactones, exerts multiple biological and therapeutic activities including anti-inflammatory and anti-cancer effects. Here, we further study the parthenolide mechanism of action using activity-based protein
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service