All Photos(1)
About This Item
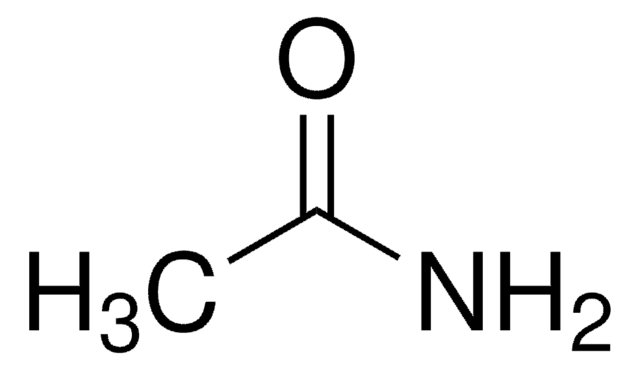
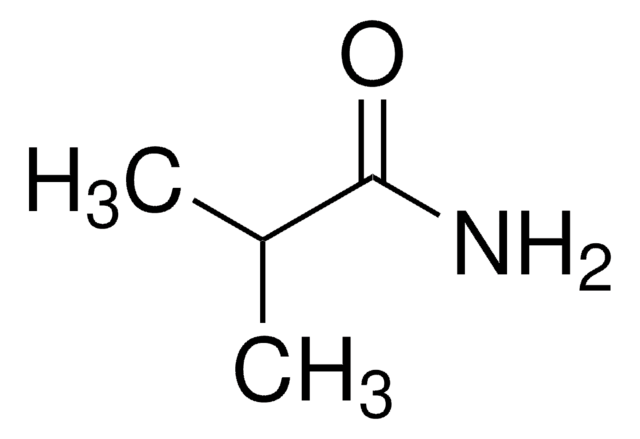
Linear Formula:
CH3CONH2
CAS Number:
Molecular Weight:
59.07
Beilstein:
1071207
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
1 mmHg ( 65 °C)
Quality Level
Assay
99%
form
sublimed
bp
221 °C (lit.)
mp
78-80 °C (lit.)
functional group
amide
SMILES string
CC(N)=O
InChI
1S/C2H5NO/c1-2(3)4/h1H3,(H2,3,4)
InChI key
DLFVBJFMPXGRIB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lingle Wang et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(6), 1937-1942 (2012-02-07)
We apply a free energy perturbation simulation method, free energy perturbation/replica exchange with solute tempering, to two modifications of protein-ligand complexes that lead to significant conformational changes, the first in the protein and the second in the ligand. The approach
Jie Guang et al.
Organic letters, 14(12), 3174-3177 (2012-06-02)
Highly enantioselective aldol reactions of acetylphosphonates and activated carbonyl compounds was realized with cinchona alkaloid derived catalysts, in which the acetylphosphonate was directly used as an enolate precursor for the first time. The aldol product obtained was converted in situ
Cristian Varela et al.
Chemistry & biology, 19(4), 498-506 (2012-04-24)
Mycolic acids are vital components of the cell wall of the tubercle bacillus Mycobacterium tuberculosis and are required for viability and virulence. While mycolic acid biosynthesis is studied extensively, components involved in mycolate transport remain unidentified. We investigated the role
A E Alves et al.
Reproduction in domestic animals = Zuchthygiene, 47(6), 1003-1008 (2012-03-06)
This study was undertaken to compare cryotolerance, in terms of viability and resumption of meiosis after warming and culture (24 and 48 h), of ex situ (isolated) and in situ (enclosed in the ovarian tissue) feline cumulus-oocyte complexes (COCs) vitrified
A Subha Mahadevi et al.
Physical chemistry chemical physics : PCCP, 13(33), 15211-15220 (2011-07-16)
Insights into the formation of hydrogen bonded clusters are of outstanding importance and quantum chemical calculations play a pivotal role in achieving this understanding. Structure and energetic comparison of linear, circular and standard forms of (acetamide)(n) clusters (n = 1-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service