43456
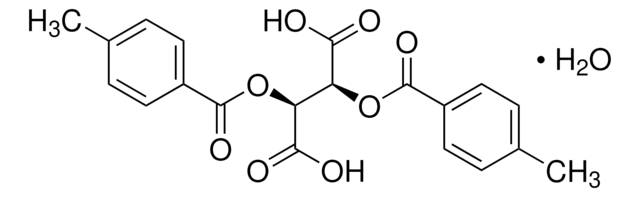
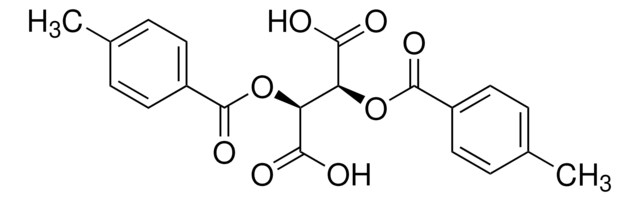
(+)-O,O′-Di-pivaloyl-D-tartaric acid
≥98.0%
Synonym(s):
(+)-DPTA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3CCO2CH(CO2H)-]2
CAS Number:
Molecular Weight:
318.32
Beilstein:
6894779
MDL number:
UNSPSC Code:
12352108
eCl@ss:
39021705
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0%
optical activity
[α]20/D +23.5±1°, c = 1.7% in dioxane
mp
127-130 °C (lit.)
functional group
carboxylic acid
ester
SMILES string
CC(C)(C)C(=O)O[C@@H]([C@H](OC(=O)C(C)(C)C)C(O)=O)C(O)=O
InChI
1S/C14H22O8/c1-13(2,3)11(19)21-7(9(15)16)8(10(17)18)22-12(20)14(4,5)6/h7-8H,1-6H3,(H,15,16)(H,17,18)/t7-,8-/m0/s1
InChI key
UFHJEZDFEHUYCR-YUMQZZPRSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(+)-O,O′-Di-pivaloyl-D-tartaric acid is a chiral protonating agent (CPA).
Other Notes
Reagent for the "deracemization" of carbonyl compounds by enantio-selective protonation of enamines or Li-enolates ; Synthesis of optically active α-amino acids
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Deracemisation par protonation enantioselective.
L. Duhamel, J.-C. Plaquevent
Tetrahedron Letters, 2285-2285 (1977)
Deracemization by enantioselective protonation. A new method for the enantiomeric enrichment of. alpha.-amino acids.
L. Duhamel, J.-C. Plaquevent
Journal of the American Chemical Society, 100, 7415-7415 (1978)
Ligand exchange in asymmetric reactions of lithium enolates: application to the deracemization of a-aminoacids.
L. Duhamel et al.
Tetrahedron Letters, 27, 4975-4975 (1986)
L. Duhamel
Bulletin de la Societe Chimique De France, II, 421-421 (1984)
Enantioselective protonations: fundamental insights and new concepts.
Duhamel L, et al.
Tetrahedron Asymmetry, 15(23) (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service