428566
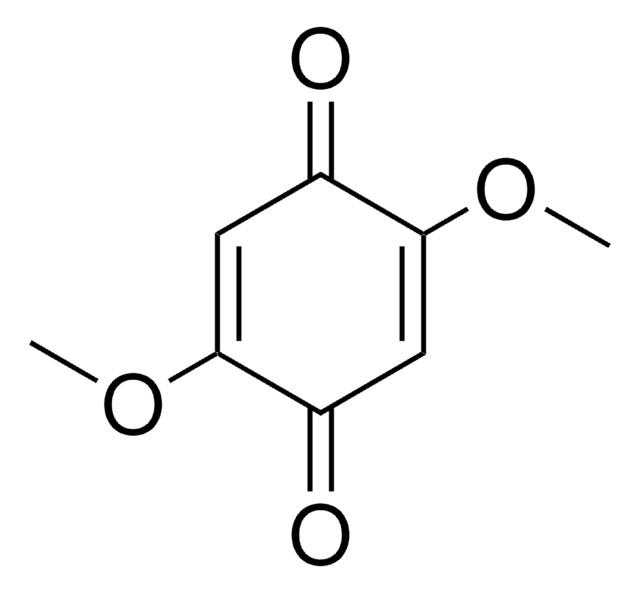
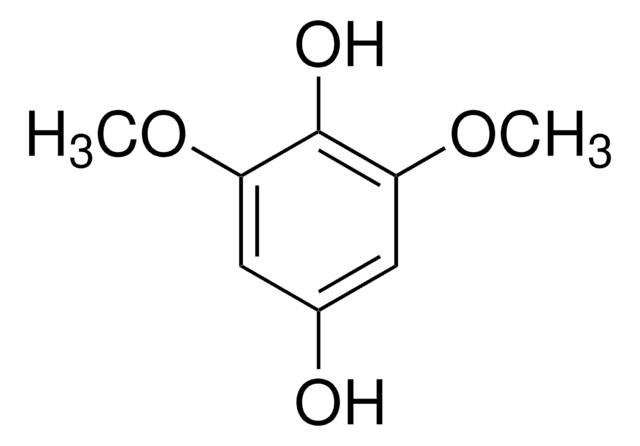
2,6-Dimethoxy-1,4-benzoquinone
97%
Synonym(s):
DMBQ
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C8H8O4
CAS Number:
Molecular Weight:
168.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
253-257 °C (dec.) (lit.)
solubility
DMSO: soluble(lit.)
functional group
ether
ketone
SMILES string
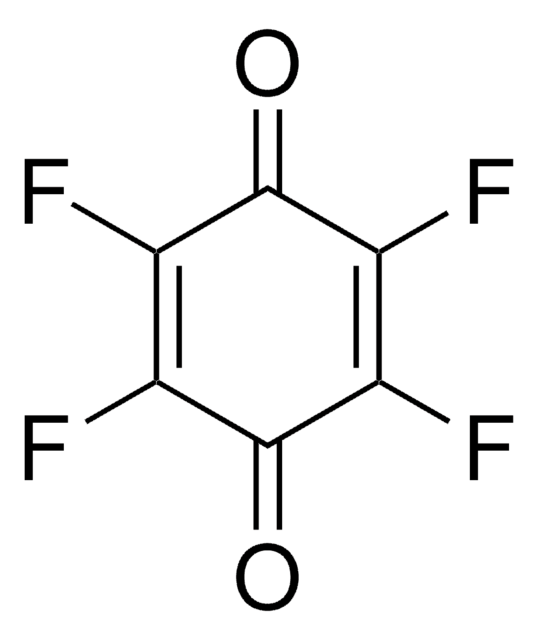
COC1=CC(=O)C=C(OC)C1=O
InChI
1S/C8H8O4/c1-11-6-3-5(9)4-7(12-2)8(6)10/h3-4H,1-2H3
InChI key
OLBNOBQOQZRLMP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Dimethoxy-1,4-benzoquinone (DBQ, 2,6-DMBQ, DMOBQ) is a 1,4-benzoquinone derivative. It is a wood allergen, has been reported to cause various skin and mucosal symptoms on exposure to wood dusts. It is formed as a product due to the activity of bacterial Azospirillum lipoferum laccase on phenolic compounds of the syringic type. DBQ is one of the components isolated from the rhizome of Gynura japonica with a potential to show anti-platelet aggregation activity in vitro. It is an anticancer agent, whose kinetics of cyclic redox transformation induced by ascorbate (AscH-) has been studied using the Clark electrode and ESR techniques. Its electrochemical reduction in acetonitrile has been studied.
Application
2,6-Dimethoxy-1,4-benzoquinone may be used in the synthesis of 2-aryl-3,5-dimethoxy-1,4-benzoquinone derivatives.
Known haustorial inducing factor.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fang-Rong Chang et al.
Journal of natural products, 65(3), 255-258 (2002-03-23)
Three new eudesmanolide sesquiterpenes, neolitacumone A-C (1-3), and one new benzylisoquinoline alkaloid, neolitacumonine (5), along with 27 known compounds were isolated from the stem bark of Neolitsea acuminatissima. The structures of compounds 1-3 and 5 were established on the basis
Lelde Krumina et al.
Environmental science & technology, 51(16), 9053-9061 (2017-07-12)
Hydroquinones are important mediators of electron transfer reactions in soils with a capability to reduce Fe(III) minerals and molecular oxygen, and thereby generating Fenton chemistry reagents. This study focused on 2,6-dimethoxy hydroquinone (2,6-DMHQ), an analogue to a common fungal metabolite
V A Roginsky et al.
Biochemistry. Biokhimiia, 63(2), 200-206 (1998-06-02)
The kinetics of cyclic redox transformation of 2,6-dimethoxy-1, 4-benzoquinone (DMOBQ)--the well-known effective anticancer agent--induced by ascorbate (AscH-) were studied in phosphate buffer, pH 7.40, at 37 degreesC using the Clark electrode and ESR techniques. The process is due to the
Anomalous behavior in the two-step reduction of quinones in acetonitrile.
Lehmann MW and Evans DH.
Journal of Electroanalytical Chemistry, 500(1), 12-20 (2001)
Quy A Ngo et al.
BMC plant biology, 13, 28-28 (2013-02-20)
Plant parasitism represents an extraordinary interaction among flowering plants: parasitic plants use a specialized organ, the haustorium, to invade the host vascular system to deprive host plants of water and nutrients. Various compounds present in exudates of host plants trigger
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service