412260
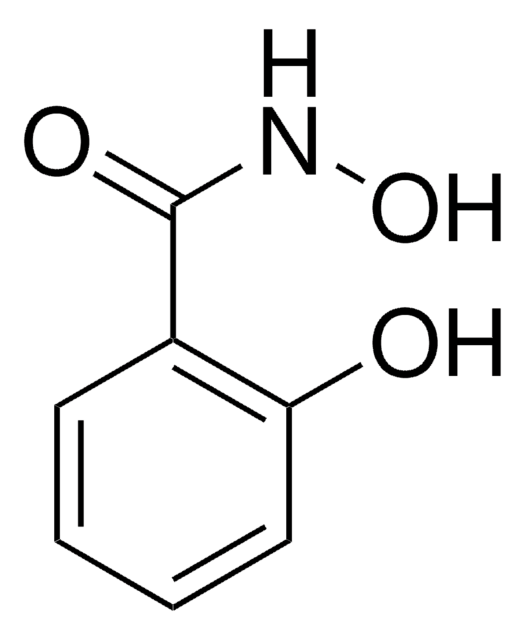
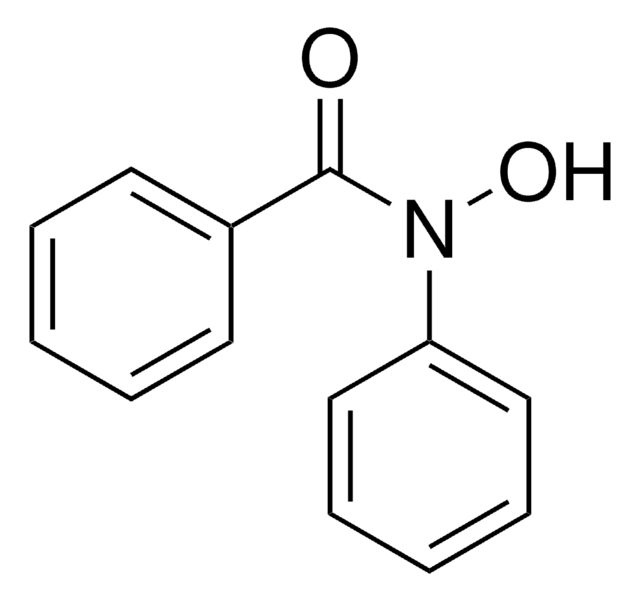
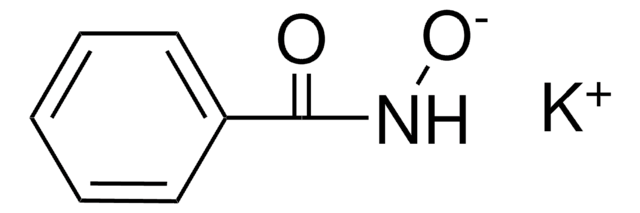
Benzhydroxamic acid
99%
Synonym(s):
N-Hydroxybenzamide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CONHOH
CAS Number:
Molecular Weight:
137.14
Beilstein:
1907585
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
126-130 °C (lit.)
solubility
1 M NaOH: soluble 50 mg/mL, clear, colorless
functional group
amine
phenyl
storage temp.
2-8°C
SMILES string
ONC(=O)c1ccccc1
InChI
1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9)
InChI key
VDEUYMSGMPQMIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Benzhydroxamic acid (BHA) reacts with BiPh3 or Bi(O(t)Bu)3 to afford novel mono- and di-anionic hydroxamato complexes, having anti-bacterial activity against Helicobacter pylori. Three-dimensional structure of recombinant horseradish peroxidase-BHA complex has been studied.
Application
Benzhydroxamic acid may be employed for the Pd-catalyzed synthesis of benzisoxazolones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Henriksen et al.
Biochemistry, 37(22), 8054-8060 (1998-06-12)
The three-dimensional structure of recombinant horseradish peroxidase in complex with BHA (benzhydroxamic acid) is the first structure of a peroxidase-substrate complex demonstrating the existence of an aromatic binding pocket. The crystal structure of the peroxidase-substrate complex has been determined to
Swetlana Gez et al.
Inorganic chemistry, 44(8), 2934-2943 (2005-04-12)
A new family of relatively stable Cr(V) complexes, [Cr(V)O(L)(2)](-) (LH(2) = RC(O)NHOH, R = Me, Ph, 2-HO-Ph, or HONHC(O)(CH(2))(6)), has been obtained by the reactions of hydroxamic acids with Cr(VI) in polar aprotic solvents. Similar reactions in aqueous solutions led
Fernando Ruy et al.
Journal of bioenergetics and biomembranes, 38(2), 129-135 (2006-10-21)
Candida parapsilosis mitochondria contain three respiratory chains: the classical respiratory chain (CRC), a secondary parallel chain (PAR) and an "alternative" oxidative pathway (AOX). We report here the existence of similar pathways in C. albicans. To observe the capacity of each
Florian Thaler et al.
Journal of medicinal chemistry, 53(2), 822-839 (2009-12-19)
The histone deacetylases (HDACs) are able to regulate gene expression, and histone deacetylase inhibitors (HDACi) emerged as a new class of agents in the treatment of cancer as well as other human disorders such as neurodegenerative diseases. In the present
Henrik R Hallingbäck et al.
Biochemistry, 45(9), 2940-2950 (2006-03-01)
The oxidation of melatonin by the mammalian myeloperoxidase (MPO) provides protection against the damaging effects of reactive oxygen species. Indole derivatives, such as melatonin and serotonin, are also substrates of the plant horseradish peroxidase (HRP), but this enzyme exhibits remarkable
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service