358959
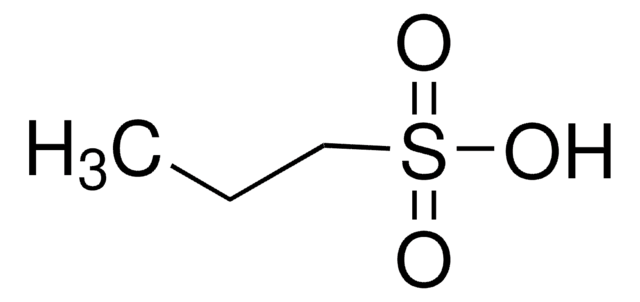
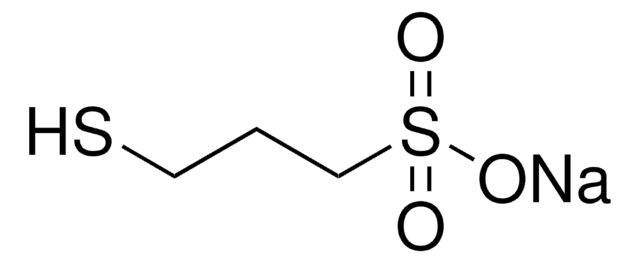
Sodium 1-propanesulfonate monohydrate
99%
Synonym(s):
1-Propanesulfonic acid sodium salt monohydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
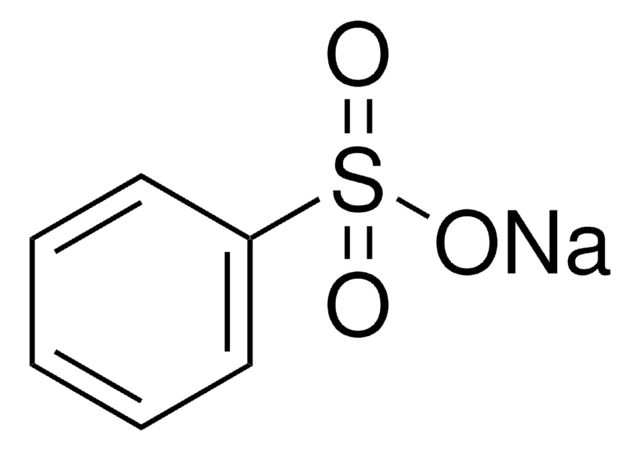
CH3CH2CH2SO3Na · H2O
CAS Number:
Molecular Weight:
164.16
Beilstein:
3714817
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
250 °C (dec.) (lit.)
solubility
water: soluble 10%, clear, colorless
functional group
sulfonic acid
SMILES string
O.[Na+].CCCS([O-])(=O)=O
InChI
1S/C3H8O3S.Na.H2O/c1-2-3-7(4,5)6;;/h2-3H2,1H3,(H,4,5,6);;1H2/q;+1;/p-1
InChI key
QBQVXXQXZXDEHE-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sodium 1-propanesulfonate monohydrate is sulfonate-containing compound. Multicollision dissociation threshold (MCDT) values for the dissociation of the anion dopant from lacto-N-difucohexaoses I for sodium 1-propanesulfonate monohydrate (1-propanesulfonic acid sodium salt monohydrate) has been evaluated.
Application
Sodium 1-propanesulfonate monohydrate (1-Propanesulfonic acid sodium salt monohydrate) is suitable for use in a study to investigate the reaction between active methyl-coenzyme M reductase (MCR) and the potent inhibitor, 3-bromopropanesulfonate by UV-visible and EPR spectroscopy and by steady-state and rapid kinetics.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A W Wong et al.
Analytical chemistry, 72(7), 1419-1425 (2000-04-14)
Alkylsulfonates are examined as anion dopants for the matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) of neutral oligosaccharides. The anion dopants allow neutral oligosaccharides to be examined in the same mixture as acidic oligosaccharides. The alkylsulfonate dopants interact strongly with the oligosaccharide
Ryan C Kunz et al.
The Journal of biological chemistry, 281(45), 34663-34676 (2006-09-13)
Methyl-coenzyme M reductase (MCR) catalyzes the final step of methanogenesis in which coenzyme B and methyl-coenzyme M are converted to methane and the heterodisulfide, CoMS-SCoB. MCR also appears to initiate anaerobic methane oxidation (reverse methanogenesis). At the active site of
Kevin G Schmitt et al.
Physical chemistry chemical physics : PCCP, 21(30), 16838-16847 (2019-07-25)
We evaluate the effect of chain length for a series of alkyl sulfonic acid additives on Cu electrodeposition by using a combination of electrochemical and Raman spectroscopic methods. Rotating disk linear sweep voltammetry revealed the influence of these additives on
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 358959-10G | |
| 358959-1G | 4061825895222 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service