295825
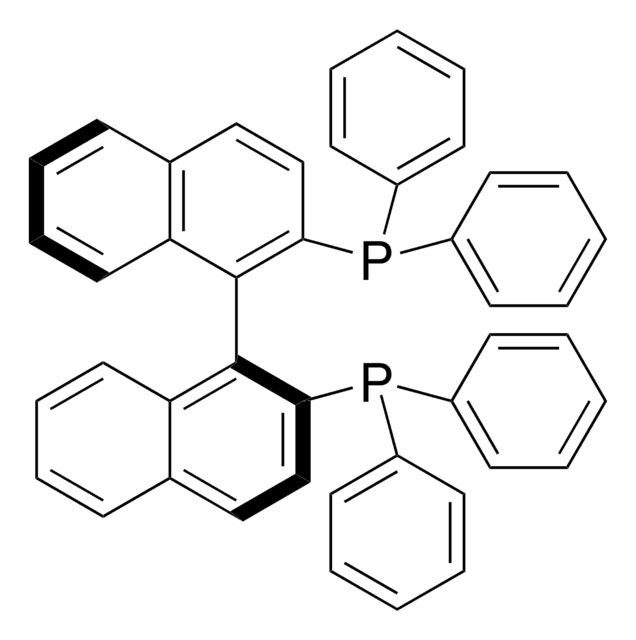
(S)-(−)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene
97%
Synonym(s):
(S)-(−)-(1,1′-Binaphthalene-2,2′-diyl)bis(diphenylphosphine), (S)-(−)-BINAP
About This Item
Recommended Products
Quality Level
Assay
97%
form
solid
optical activity
[α]19/D −233°, c = 0.3 in toluene
optical purity
ee: 99% (HPLC)
mp
238-240 °C (lit.)
functional group
phosphine
InChI
1S/C44H32P2/c1-5-19-35(20-6-1)45(36-21-7-2-8-22-36)41-31-29-33-17-13-15-27-39(33)43(41)44-40-28-16-14-18-34(40)30-32-42(44)46(37-23-9-3-10-24-37)38-25-11-4-12-26-38/h1-32H
InChI key
MUALRAIOVNYAIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ligand used in a palladium-catalyzed, asymmetric, tandem Heck reaction-carbanion capture process leading to a synthesis of a tricyclic sesquiterpene. Also used in a ruthenium-catalyzed asymmetric hydrogenation of α,β-unsaturated acids.
- Enantioselective and diastereoselective unpoled carbonyl allylation
- Syntehsis of organophophine oxides as anittumor agents
- SN2 halogenation of hydroxy groups
- Synthesis of BINAP complexes
- Studies of conformational flexibility of BINAP chelates
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article concerning BINAP/SEGPHOS® Ligands and Complexes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service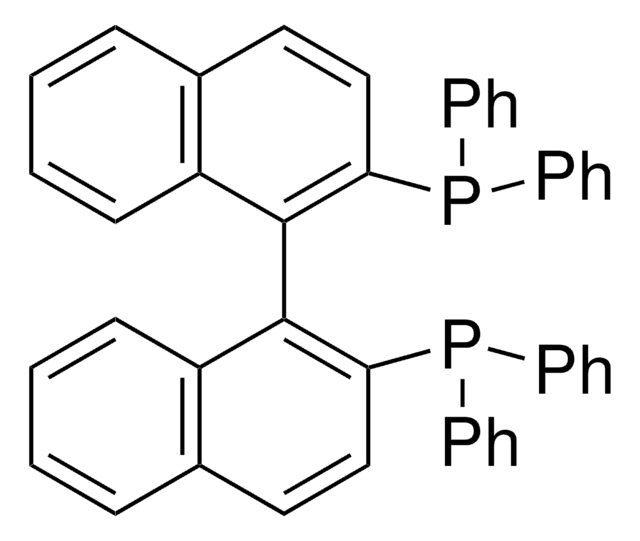



![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)