258881
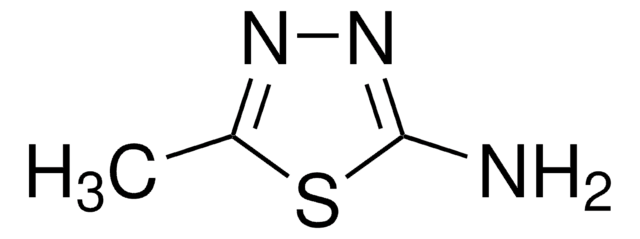
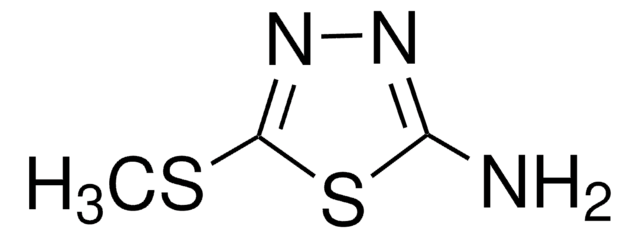
2-Amino-1,3,4-thiadiazole
97%
Synonym(s):
1,3,4-Thiadiazol-2-amine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C2H3N3S
CAS Number:
Molecular Weight:
101.13
Beilstein:
107135
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
188-191 °C (dec.) (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless
SMILES string
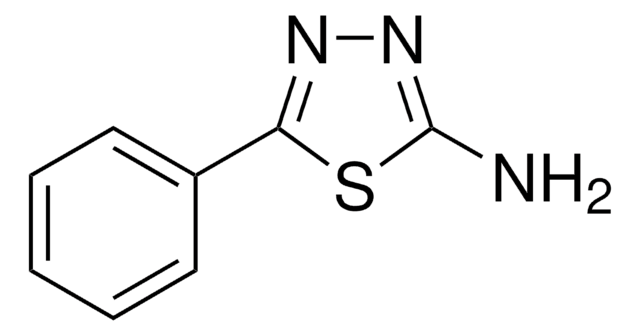
Nc1nncs1
InChI
1S/C2H3N3S/c3-2-5-4-1-6-2/h1H,(H2,3,5)
InChI key
QUKGLNCXGVWCJX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-1,3,4-thiadiazole (donor) form charge transfer complexes with 2,3-dichloro-5,6-dicyano-p-benzoquinone, p-chloranil, o-chloranil, p-bromanil and chloranilic acid (acceptors). Effects of 2-amino-1,3,4-thiadiazole [aminothiadiazole (NSC 4728)] on purine and pyrimidine ribonucleotide pools of L1210 ascites cells in vivo has been reported.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Palraj Kalimuthu et al.
Bioelectrochemistry (Amsterdam, Netherlands), 79(2), 168-172 (2010-04-23)
We report the selective determination of homocysteine (HCY) in the presence of one of the very important interferents, ascorbic acid (AA) using electropolymerized film of 2-amino-1,3,4-thiadiazole (ATD) modified glassy carbon electrode (GCE) at physiological pH for the first time. An
Andrea Cuconati et al.
PloS one, 8(1), e54595-e54595 (2013-01-29)
Hepatocellular carcinoma (HCC) is the third most common cause of cancer fatalities worldwide, with limited treatment options and five year survival rates of between <5 and 15%. To address this medical need, we conducted a screen of a drug-like small
R F Asbury et al.
American journal of clinical oncology, 9(4), 334-336 (1986-08-01)
Thirty-two evaluable patients with advanced epithelial ovarian cancer were treated with aminothiadiazole at a dosage of 125 mg/m2 weekly. Two patients had partial responses, 12 had stable disease, 16 had increasing disease, and two were inevaluable for response. Aminothiadiazole used
R F Asbury et al.
American journal of clinical oncology, 10(5), 380-382 (1987-10-01)
The Eastern Cooperative Oncology Group (ECOG) studied 29 patients with advanced measurable colon cancer who were treated with Aminothiadiazole (NSC #4728) 125 mg/m2 intravenously. Allopurinol 300 mg daily was taken by all patients during treatment. Three patients (12%) demonstrated partial
Małgorzata Juszczak et al.
Folia histochemica et cytobiologica, 49(3), 436-444 (2011-11-01)
The 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazole set are well known compounds with interesting in vitro and in vivo anti-cancer profiles. The aim of this study was an in vitro evaluation of the anti-cancer activity of a new synthesized aminothiadiazole derivative 2-(3-chlorophenyloamino)-5-(2,4-dihydroxyphenyl)- -1,3,4-thiadiazole 4ClABT. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service