All Photos(1)
About This Item
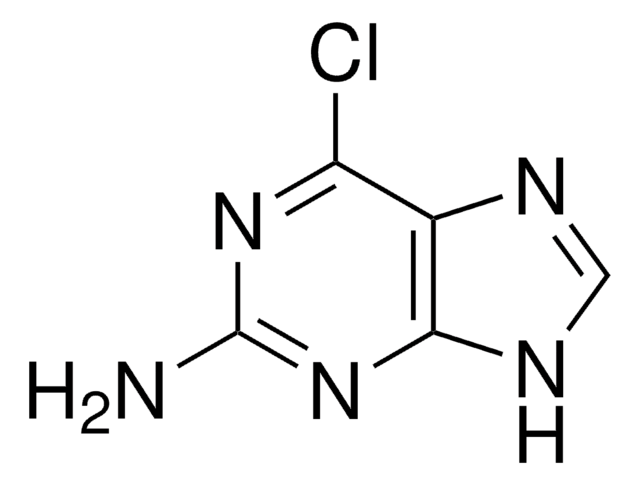
Empirical Formula (Hill Notation):
C5H6N6
CAS Number:
Molecular Weight:
150.14
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
mp
>300 °C (lit.)
SMILES string
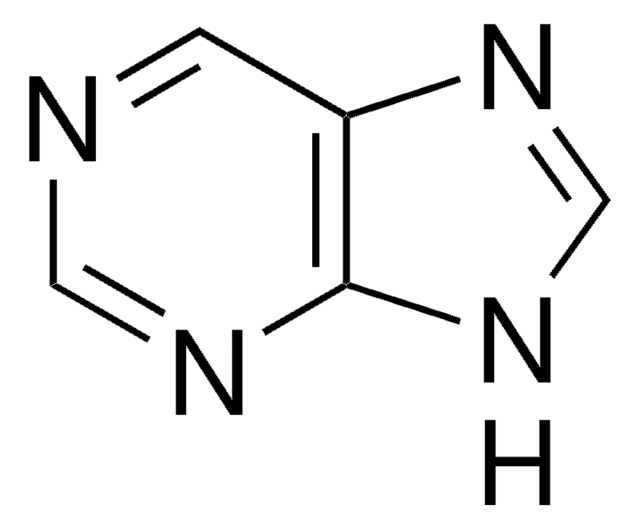
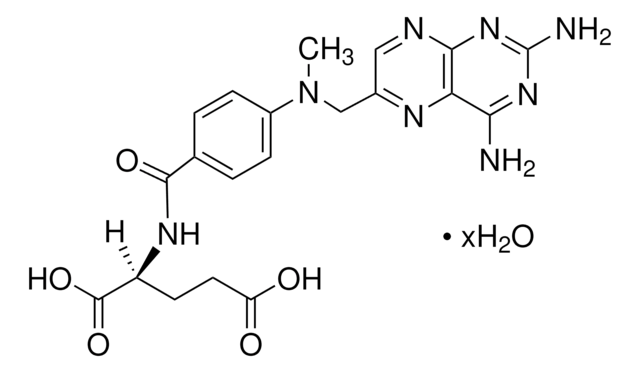
Nc1nc(N)c2nc[nH]c2n1
InChI
1S/C5H6N6/c6-3-2-4(9-1-8-2)11-5(7)10-3/h1H,(H5,6,7,8,9,10,11)
InChI key
MSSXOMSJDRHRMC-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Søren Lindemose et al.
Nucleic acids research, 36(14), 4797-4807 (2008-07-26)
The DNA interaction of the Escherichia coli cyclic AMP receptor protein (CRP) represents a typical example of a dual recognition mechanism exhibiting both direct and indirect readout. We have dissected the direct and indirect components of DNA recognition by CRP
V Krishnakumar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 69(1), 8-17 (2007-05-01)
FT-IR and FT-Raman spectra of 2,6-diamino purine (DAP) and 6-methoxy purine (MP) have been recorded in the regions of 4000-400cm(-1) and 3500-100cm(-1), respectively. The spectra were interpreted with the aid of normal coordinate analysis following full structure optimizations and force
Miguel A Galindo et al.
Inorganic chemistry, 48(23), 11085-11091 (2009-10-28)
Alkyldiamine-tethered derivatives of 2,6-diaminopurine, ethylenediamine-N9-propyl-2,6-diaminopurine, L1, and ethylenediamine-N9-ethyl-2,6-diaminopurine, L2, react with Pd(II) to give N3-coordinated complexes. However, the exact nature of the resulting complex is dependent on the reaction conditions. With PdCl(2)(MeCN)(2) in MeCN/H(2)O the expected [PdCl(N3-2,6-DAP-alkyl-en)](+) complex, 1, is
Elizabeth Mburu et al.
The journal of physical chemistry. A, 112(48), 12485-12491 (2008-11-07)
Several excited singlet electronic states of purine nucleobases and related derivatives have been calculated using high-level multireference perturbation theory methods. Purine derivatives with one or two amino or carbonyl groups substituted at positions C(2) and/or C(6) of the purine ring
Kiyohiko Kawai et al.
Journal of the American Chemical Society, 132(2), 627-630 (2009-12-18)
A positive charge migrates along DNA mainly via a series of short-range charge transfer (CT) processes between G-C base pairs, which have relatively high HOMO levels. As such, the CT efficiency sharply decreases with the insertion of A-T base pairs
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service