237647
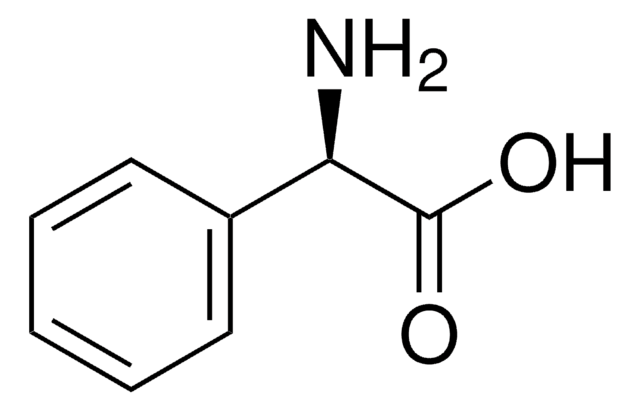
L−(+)-α-Phenylglycine
99%
Synonym(s):
(S)-(+)-2-Phenylglycine, S-(+)-α-Aminophenylacetic acid, L-2-Phenylglycine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
151.16
Beilstein:
2208675
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
optical activity
[α]20/D +155°, c = 1 in 1 M HCl
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
application(s)
peptide synthesis
SMILES string
N[C@H](C(O)=O)c1ccccc1
InChI
1S/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)/t7-/m0/s1
InChI key
ZGUNAGUHMKGQNY-ZETCQYMHSA-N
Application
Chiral starting material.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Henry Kurniawan et al.
Cell metabolism, 31(5), 920-936 (2020-03-28)
Regulatory T cells (Tregs) maintain immune homeostasis and prevent autoimmunity. Serine stimulates glutathione (GSH) synthesis and feeds into the one-carbon metabolic network (1CMet) essential for effector T cell (Teff) responses. However, serine's functions, linkage to GSH, and role in stress responses in
Kuoxi Xu et al.
Chirality, 24(8), 646-651 (2012-05-24)
The triazine-based bisbinaphthyl crown ethers oxacalix[2]arene[2]bisbinaphthes R-1, R-2, R-3 and S-1, S-2, S-3 were synthesized. The interactions of these compounds with various α-aminocarboxylic acid anions were studied. The crown ethers were found to carry out highly enantioselective fluorescent recognition of
Hyung Min Kim et al.
The Journal of chemical physics, 128(18), 184313-184313 (2008-06-06)
We investigated the conformational structures of L-phenylglycine in the gas phase by photoionization and double resonance spectroscopy techniques as well as high-level ab initio calculations. The UV-UV and IR-UV double resonance spectroscopy suggested that there exists only one conformer that
Caroline Haurena et al.
The Journal of organic chemistry, 75(8), 2645-2650 (2010-03-23)
A range of alpha-amino esters has been synthesized in good to high yields using a straightforward three-component reaction among preformed or in situ generated aromatic or benzylic organozinc reagents, primary or secondary amines, and ethyl glyoxylate. The procedure, which is
Choedchai Saehuan et al.
Biochimica et biophysica acta, 1770(11), 1585-1592 (2007-10-06)
Following induction with D-phenylglycine both d-phenylglycine aminotransferase activity and benzoylformate decarboxylase activity were observed in cultures of Pseudomonas stutzeri ST-201. Induction with benzoylformate, on the other hand, induced only benzoylformate decarboxylase activity. Purification of the benzoylformate decarboxylase, followed by N-terminal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service