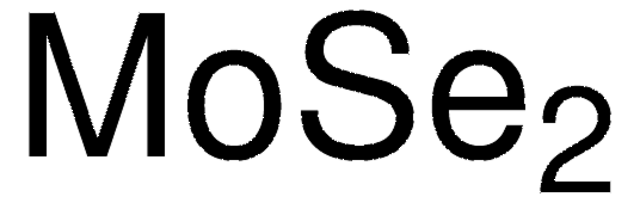234842
Molybdenum(IV) sulfide
powder, <2 μm, 98%
Synonym(s):
Molybdenum disulfide
About This Item
Recommended Products
Quality Level
Assay
98%
form
powder
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
particle size
<2 μm
density
5.06 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
greener alternative category
, Enabling
SMILES string
S=[Mo]=S
InChI
1S/Mo.2S
InChI key
CWQXQMHSOZUFJS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Developed in the last several years, fluorescence quenching microscopy (FQM) has enabled rapid, inexpensive, and high-fidelity visualization of two-dimensional (2D) materials such as graphene-based sheets and MoS2.
An article concerning self-propagating reactions induced by mechanical alloying, presented by Sigma-Aldrich.com.
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
The production of hydrogen by catalytic water splitting is important for a wide range of industries including renewable energy petroleum refining and for the production of methanol and ammonia in the chemical industry.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service