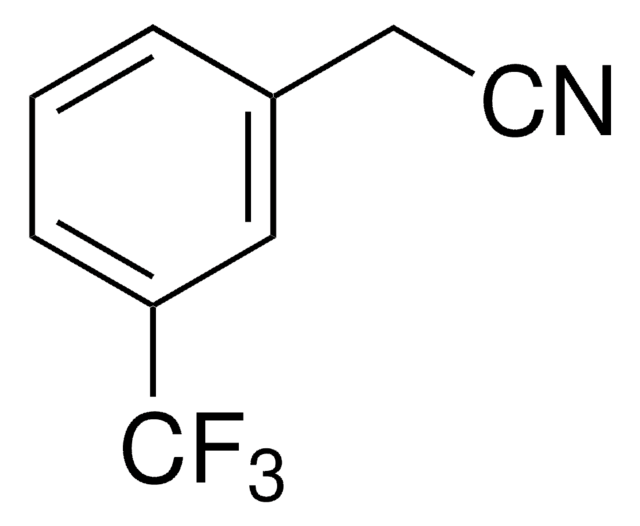
233161
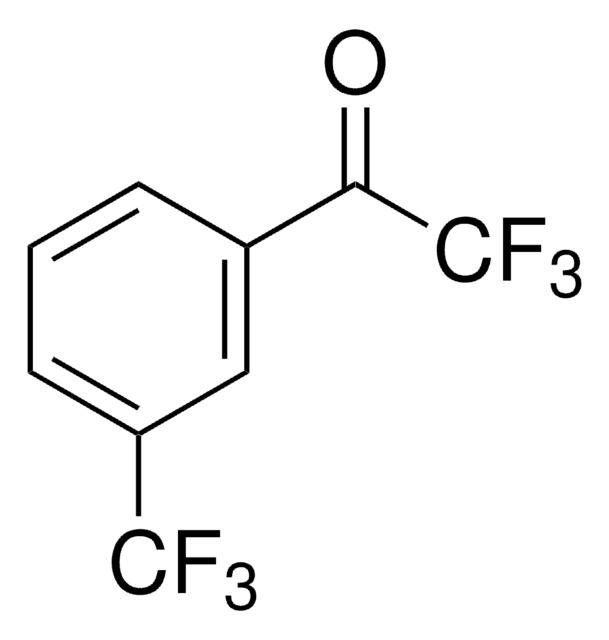
3′-(Trifluoromethyl)acetophenone
99%
Synonym(s):
3-Acetylbenzotrifluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3C6H4COCH3
CAS Number:
Molecular Weight:
188.15
Beilstein:
640151
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.4611 (lit.)
bp
198-200 °C (lit.)
density
1.235 g/mL at 25 °C (lit.)
functional group
fluoro
ketone
SMILES string
CC(=O)c1cccc(c1)C(F)(F)F
InChI
1S/C9H7F3O/c1-6(13)7-3-2-4-8(5-7)9(10,11)12/h2-5H,1H3
InChI key
ABXGMGUHGLQMAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Asymmetric catalytic addition of ethyl groups to 3′-(trifluoromethyl)acetophenone catalyzed by ligands derived from trans-1,2-diaminocyclohexane and camphor sulfonyl chloride has been reported. Phenylation of 3′-(trifluoromethyl)acetophenone in the presence of dihydroxy bis(sulfonamide) ligand (enantioselective catalyst), titanium tetraisopropoxide and diphenylzinc has been investigated.
Application
3′-(Trifluoromethyl)acetophenone has been used in a key step during the preparation of a commercial fungicide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Serafino Gladiali et al.
Chemical Society reviews, 35(3), 226-236 (2006-03-01)
Hydrogen transfer reduction processes are attracting increasing interest from synthetic chemists in view of their operational simplicity and high selectivity. In this tutorial review the most significant advances recently achieved in the stereoselective reduction of unsaturated organic compounds catalyzed by
Celina García et al.
Journal of the American Chemical Society, 124(37), 10970-10971 (2002-09-13)
Many catalysts will promote the asymmetric addition of alkylzinc reagents to aldehydes. In contrast, there are no reports of additions to ketones that are both general and highly enantioselective. We describe herein a practical catalytic asymmetric addition of ethyl groups
Celina García et al.
Organic letters, 5(20), 3641-3644 (2003-09-26)
[reaction: see text] The catalytic asymmetric addition of phenyl groups from diphenylzinc to ketones is reported. The catalyst, generated from a dihydroxy bis(sulfonamide) ligand and titanium tetraisopropoxide, gives good to excellent enantioselectivities with a range of substrates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service