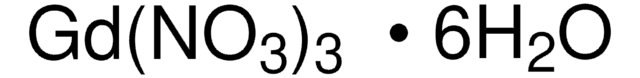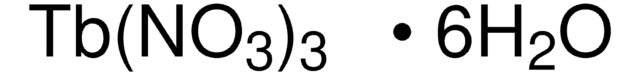211591
Gadolinium(III) nitrate hexahydrate
crystals and lumps, 99.9% trace metals basis
Synonym(s):
Gadolinium hexahydrate trinitrate
About This Item
Recommended Products
Quality Level
Assay
99.9% trace metals basis
form
crystals and lumps
reaction suitability
reagent type: catalyst
core: gadolinium
impurities
≤1500.0 ppm Trace Rare Earth Analysis
mp
91 °C (lit.)
SMILES string
[Gd+3].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Gd.3NO3.6H2O/c;3*2-1(3)4;;;;;;/h;;;;6*1H2/q+3;3*-1;;;;;;
InChI key
XWFVFZQEDMDSET-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a precursor to synthesize Gadolinium oxide (Gd2O3) nanoparticles for various applications such as photocatalysis and biomedical imaging.
- As a dopant to prepare ZnO nanoparticles with enhanced room temperature ferromagnetism.
- To prepare Ceria (CeO2)-based ceramics doped electrolytes for intermediate temperature solid oxide fuel cells (IT-SOFCs) .
- As a raw material for the synthesis of Eu3+-Activated KGd2F7 red-emitting nanoparticles for white light-emitting diode.
- In addition,it is also reported to producegreen light emission of Er3+-activated single-phased GdAlO3 phosphors for lighting applications.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service