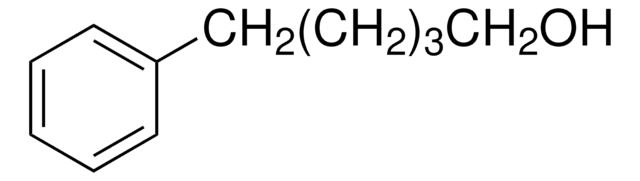All Photos(1)
About This Item
Linear Formula:
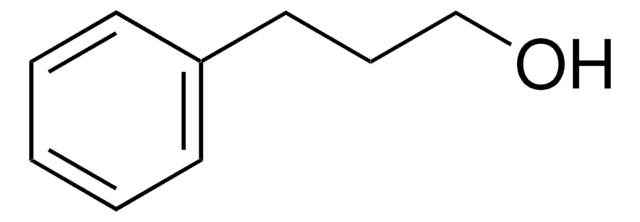
C6H5(CH2)4OH
CAS Number:
Molecular Weight:
150.22
Beilstein:
2042122
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.521 (lit.)
bp
140-142 °C/14 mmHg (lit.)
density
0.984 g/mL at 20 °C (lit.)
SMILES string
OCCCCc1ccccc1
InChI
1S/C10H14O/c11-9-5-4-8-10-6-2-1-3-7-10/h1-3,6-7,11H,4-5,8-9H2
InChI key
LDZLXQFDGRCELX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Phenyl-1-butanol is oxidized by ceric ammonium nitrate in acetonitrile to afford 2-phenyltetrahydrofuran. It undergoes cyclization in presence of phosphoric acid at high temperature to yield tetralin.
Application
4-Phenyl-1-butanol was used in synthesis of NK105, a paclitaxel-incorporating micellar nanoparticle formulation.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Hamaguchi et al.
British journal of cancer, 92(7), 1240-1246 (2005-03-24)
Paclitaxel (PTX) is one of the most effective anticancer agents. In clinical practice, however, high incidences of adverse reactions of the drug, for example, neurotoxicity, myelosuppression, and allergic reactions, have been reported. NK105, a micellar nanoparticle formulation, was developed to
New Friedel-Crafts chemistry. XIX. Cyclialkylations of some phenylalkanols.
Khalaf AA and Roberts RM.
The Journal of Organic Chemistry, 34(11), 3571-3574 (1969)
Fabrice Gritti et al.
Journal of chromatography. A, 1524, 108-120 (2017-10-11)
A twin-column recycling separation process (TCRSP) is assembled and used to generate higher speed and/or higher resolution levels than those of the usual non-recycling process at the same back pressure. It enables the users to solve very challenging separation problems
Cyclic ether formation in oxidations of primary alcohols by cerium (IV). Reactions of 5-phenyl-1-pentanol, 4-phenyl-1-butanol, and 3-phenyl-1-propanol with ceric ammonium nitrate.
Doyle MP, et al.
The Journal of Organic Chemistry, 40(10), 1454-1456 (1975)
Min Kyung Song et al.
Journal of agricultural and food chemistry, 67(7), 2028-2035 (2019-01-31)
Caffeic acid phenethyl ester (CAPE) is an ester of a hydroxycinnamic acid (phenylpropanoid) and a phenylethanoid (2-phenylethanol; 2-PE), which has long been used in traditional medicine. Here, we synthesized 54 hydroxycinnamic acid-phenylethanoid esters by feeding 64 combinations of hydroxycinnamic acids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service