181528
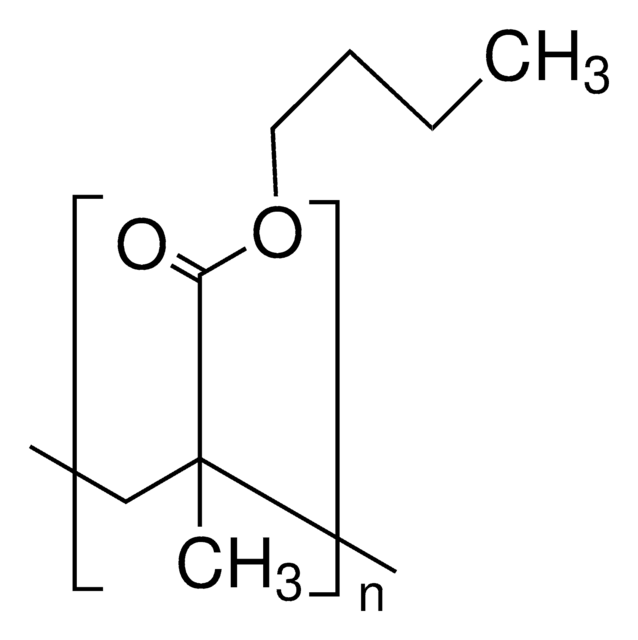
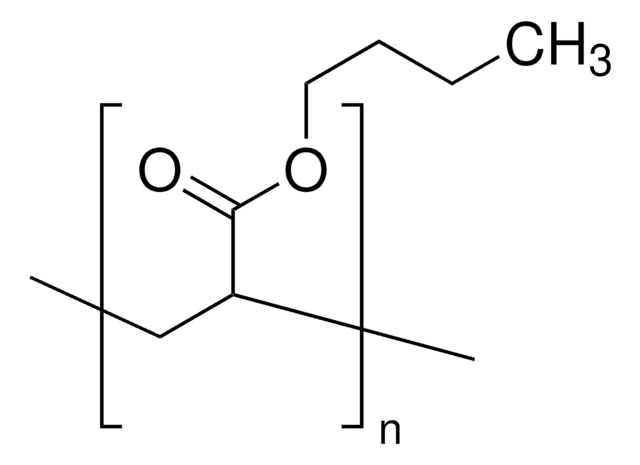
Poly(butyl methacrylate)
inherent viscosity 0.470-0.560 dL/g
Synonym(s):
PBMA
About This Item
Recommended Products
form
powder
mol wt
200000
refractive index
n20/D 1.483
inherent viscosity
0.470-0.560 dL/g
density
1.07 g/mL at 25 °C (lit.)
SMILES string
CCCCOC(=O)C(C)=C
InChI
1S/C8H14O2/c1-4-5-6-10-8(9)7(2)3/h2,4-6H2,1,3H3
InChI key
SOGAXMICEFXMKE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
It can be used to prepare thermoresponsive microfibers with excellent mechanical properties. PBMA-containing microfibers can be used as temperature-modulated cell separation materials.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Self-assembled monolayers (SAMs) have attracted enormous interest for a wide variety of applications in micro- and nano-technology. In this article, we compare the benefits of three different classes of SAM systems (alkylthiolates on gold.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service