166502
Glyoxal sodium bisulfite addition compound hydrate
Synonym(s):
1,2-Dihydroxy-1,2-ethanedisulfonic acid disodium salt hydrate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
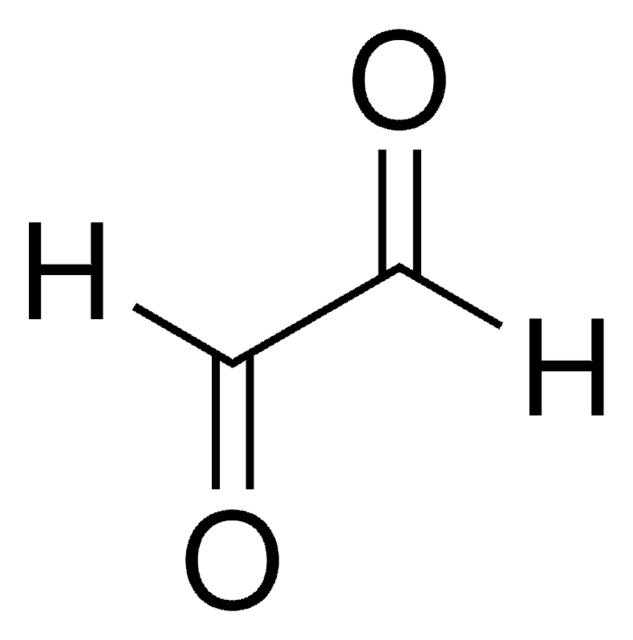
Linear Formula:
[-CH(OH)SO3Na]2 · xH2O
CAS Number:
Molecular Weight:
266.16 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
mp
193 °C (dec.) (lit.)
SMILES string
O.[Na+].[Na+].OC(C(O)S([O-])(=O)=O)S([O-])(=O)=O
InChI
1S/C2H6O8S2.2Na.H2O/c3-1(11(5,6)7)2(4)12(8,9)10;;;/h1-4H,(H,5,6,7)(H,8,9,10);;;1H2/q;2*+1;/p-2
InChI key
JQYGKJGYGOFFIK-UHFFFAOYSA-L
General description
Convenient, nonaqueous form of glyoxal
Application
Glyoxal sodium bisulfite addition compound hydrate was used to convert N-benzyl-2,3,4-trimethoxyaniline to sodium 5,6,7-trimethoxy-1-benzylindolyl-2-sulfite. It was used in the synthesis of 6-hydroxyquinoxaline.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mescaline Analogs. VIII. Substituted 5-Methoxy-and 5, 6, 7-Trimethoxyindoles.
Benington F, et al.
The Journal of Organic Chemistry, 23(1), 19-23 (1958)
404. Quinoxaline N-oxides. Part V. Further Bz-substituted derivatives.
Silk JA.
Journal of the Chemical Society, 2058-2063 (1956)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service