125954
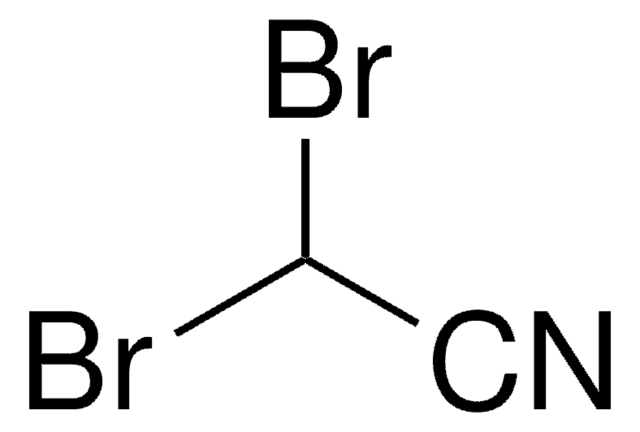
Dichloroacetonitrile
98%
Synonym(s):
2,2-Dichloroacetonitrile, Dichloromethyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl2CHCN
CAS Number:
Molecular Weight:
109.94
Beilstein:
1739029
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.44 (lit.)
bp
110-112 °C (lit.)
density
1.369 g/mL at 25 °C (lit.)
SMILES string
ClC(Cl)C#N
InChI
1S/C2HCl2N/c3-2(4)1-5/h2H
InChI key
STZZWJCGRKXEFF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Dichloroacetonitrile can be used as a reactant to prepare:
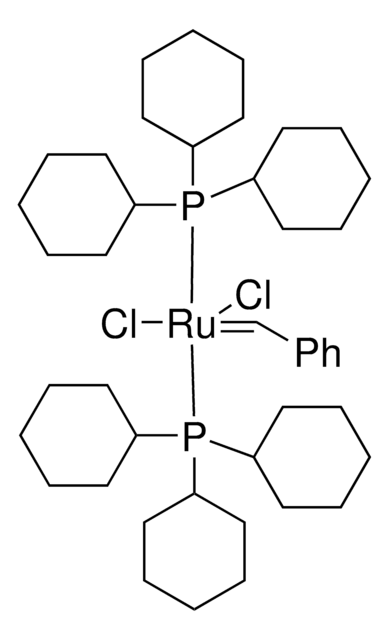
- Chiral α, α-dichloro-β-aminonitriles via Pd-catalyzed enantioselective Mannich-type reaction with imines.
- α, α-dialkyl-substituted nitriles by an alkylation reaction with trialkylboranes in the presence of phenoxide base as a base.
- Halogenated pyridines via copper-catalyzed reaction with methacrolein.
- α,α-dichloro-β-hydroxy nitriles by condensation reaction with aldehydes and ketones in the presence of an alkoxide base.
- Selenium heterocycle derivatives via Diels–Alder cyclization with selenoaldehydes.
- Dichloroacetonitrile can also be used to develop an efficient method for the extraction and determination of common volatile halogenated disinfection by-products using the static headspace technique coupled with gas chromatography-mass spectrometry.
Biochem/physiol Actions
Dichloroacetonitrile is direct-acting mutagen and induces DNA strand breaks in cultured human lymphoblastic cells. It induces apoptosis or necrosis in murine macrophage cell line via reactive oxygen intermediates-mediated oxidative mechanisms of cellular damage.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
96.8 °F - closed cup
Flash Point(C)
36 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Isabel Montesinos et al.
Journal of chromatography. A, 1310, 113-120 (2013-09-03)
A simple and efficient method has been developed for the extraction and determination of sixteen common volatile halogenated disinfection by-products (DBPs) using the static headspace (HS) technique coupled with gas chromatography-mass spectrometry (GC-MS). The DBPs determined included trihalomethanes (THMs), halonitromethanes
M K Smith et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 12(4), 765-772 (1989-05-01)
Dichloroacetonitrile (DCAN), a by-product of drinking water disinfection formed by reaction of chlorine with background organic materials, was evaluated for its developmental effects in pregnant Long-Evans rats. Animals were dosed by oral intubation on Gestation Days 6-18 (plug = 0)
Dichloroacetonitrile
GL Bundy
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Speciation of common volatile halogenated disinfection by-products in tap water under different oxidising agents
Montesinos I and Gallego M
Journal of Chromatography A, 1310, 113-120 (2013)
M R Roby et al.
Environmental health perspectives, 69, 215-220 (1986-11-01)
The excretion and tissue distribution of [1-14C]dichloroacetonitrile and [2-14C]dichloroacetonitrile were studied in male Fischer 344 rats and male B6C3F1 mice. Three dose levels of dichloroacetonitrile (DCAN) (0.2, 2, or 15 mg/kg) were administered to rats and two dose levels of
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 125954-100G | |
| 125954-1KG | |
| 125954-25G | 4061838723772 |
| 125954-500G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service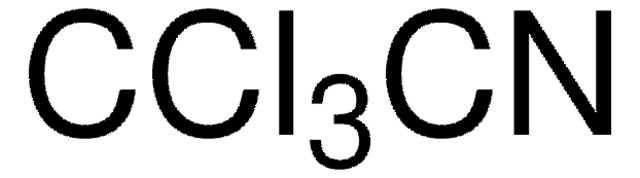



![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)