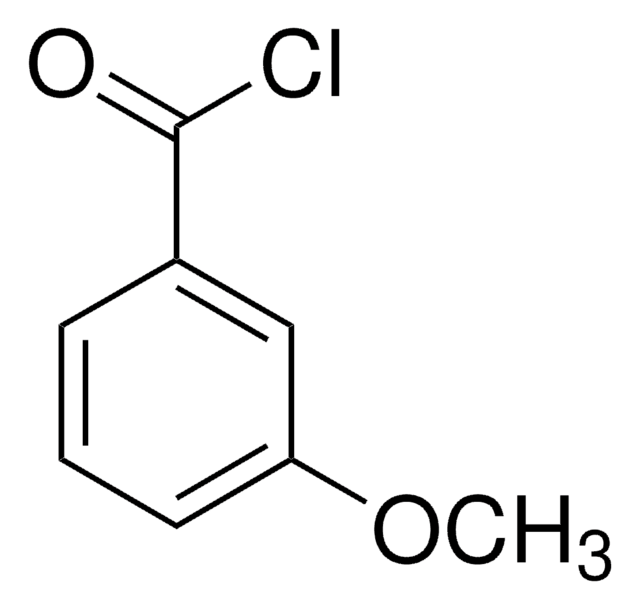
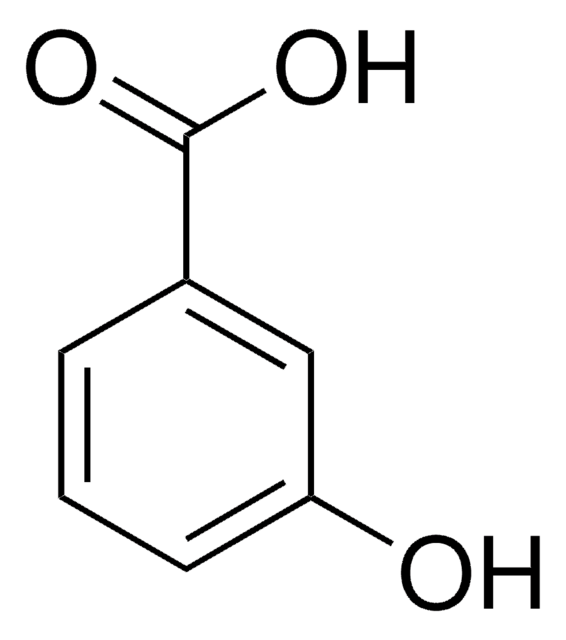
117714
3-Methoxybenzoic acid
ReagentPlus®, 99%
Synonym(s):
m-Anisic acid, m-Methylsalicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3OC6H4CO2H
CAS Number:
Molecular Weight:
152.15
Beilstein:
508838
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
170-172 °C/10 mmHg (lit.)
mp
105-107 °C (lit.)
solubility
95% ethanol: soluble 50 mg/mL, clear, colorless to faintly yellow
functional group
carboxylic acid
SMILES string
COc1cccc(c1)C(O)=O
InChI
1S/C8H8O3/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H,9,10)
InChI key
XHQZJYCNDZAGLW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Methoxybenzoic acid is an important intermediate in the synthesis of natural products.
Application
3-Methoxybenzoic acid was used in the synthesis and characterization of 3-methoxybenzoates of europium (III) and gadolinium (III). It was used in conversion of aromatic carboxylic acids into methyl esters and reduction to the corresponding primary alcohols using a sodium borohydride-THF-methanol system.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, characterization and thermal behaviour of solid-state compounds of europium (III) and gadolinium (III) 3-methoxybenzoate.
Dametto PR, et al.
Journal of Thermal Analysis and Calorimetry, 97(2), 765-768 (2009)
Sodium borohydride reduction of aromatic carboxylic acids via methyl esters.
Saeed A and Ashraf Z.
Journal of Chemical Sciences (Bangalore), 118(5), 419-423 (2006)
N Dodoff et al.
Journal of inorganic biochemistry, 54(3), 221-233 (1994-05-15)
The complexes [Pt(bah)2X2], [Pt(NH3)(bah)Cl2].0.5H2O, [Pt(mbah)2X2], and [Pt(NH3)(mbah)Cl2] (bah = benzoic acid hydrazide, mbah = 3-methoxybenzoic acid hydrazide; X = Cl, Br, I) have been prepared and characterized by elemental analysis, electric conductivity, IR, 1H NMR, and electronic spectra. A cis-square
K A DeWeerd et al.
Applied and environmental microbiology, 54(5), 1237-1242 (1988-05-01)
O-methyl substituents of aromatic compounds can provide C1 growth substrates for facultative and strict anaerobic bacteria isolated from diverse environments. The mechanism of the bioconversion of methoxylated benzoic acids to the hydroxylated derivatives was investigated with a model substrate and
Thi-Huu Nguyen et al.
Organic letters, 7(12), 2445-2448 (2005-06-04)
[reaction: see text] If employed in THF at 0 degrees C, LTMP metalates meta-anisic acid at the doubly activated position. In contrast, n-BuLi/t-BuOK deprotonates position C-4 preferentially at low temperature. Functionalization at C-6 requires protection of the C-2 site beforehand.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![Benzo[a]fluorenone BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/881/090/eae85258-97ed-4de7-90c1-c0e0e495552e/640/eae85258-97ed-4de7-90c1-c0e0e495552e.png)